
1. Glutaric Acid, Calcium Salt
2. Glutaric Acid, Copper(2+) Salt (1:1)
3. Glutaric Acid, Disodium Salt
4. Glutaric Acid, Ion(1-)
5. Glutaric Acid, Monosodium Salt
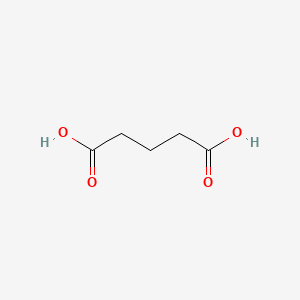
1. Pentanedioic Acid
2. 110-94-1
3. 1,5-pentanedioic Acid
4. Glutarate
5. 1,3-propanedicarboxylic Acid
6. Pentandioic Acid
7. N-pyrotartaric Acid
8. Propane-1,3-dicarboxylic Acid
9. Glutarsaeure
10. Chebi:17859
11. Hsdb 5542
12. Nsc 9238
13. Einecs 203-817-2
14. Unii-h849f7n00b
15. Brn 1209725
16. Dtxsid2021654
17. Ai3-24247
18. H849f7n00b
19. Nsc-9238
20. Mfcd00004410
21. Dtxcid401654
22. Nsc9238
23. 4-02-00-01934 (beilstein Handbook Reference)
24. 1,3-pentanedioic Acid (rifm)
25. 68603-87-2
26. 68937-69-9
27. Cas-110-94-1
28. Adipic Acid Impurity A (ep Impurity)
29. Adipic Acid Impurity A [ep Impurity]
30. Pentandioate
31. Abacavir Related
32. 1czc
33. 1,5-pentanedioate
34. Glutaric Acid, 99%
35. 4lh3
36. 1,3-propanedicarboxylate
37. Trimethylenecarboxylic Acid
38. Wln: Qv3vq
39. Pentanedioate;glutaric Acid
40. Bmse000406
41. Glutaric Acid [mi]
42. Glutaric Acid And Anhydride
43. Schembl7414
44. Glutaric Acid [hsdb]
45. Glutaric Acid [inci]
46. Pentanedioic Acid Glutaric Acid
47. Chembl1162495
48. Tox21_202448
49. Tox21_302871
50. Bdbm50485550
51. S3152
52. Akos000118800
53. Cs-w009536
54. Db03553
55. Hy-w008820
56. Ncgc00249226-01
57. Ncgc00256456-01
58. Ncgc00259997-01
59. As-13132
60. Bp-21143
61. Sy029948
62. G0069
63. G0245
64. Ns00003673
65. En300-17991
66. C00489
67. D70283
68. A802271
69. Q409622
70. Glutaric Acid (ca. 50% In Water, Ca. 4.3mol/l)
71. J-011915
72. Q-201163
73. Z57127454
74. 78fa13bf-e0c0-4efc-948c-534cf45044e3
75. F2191-0242
76. Glutaric Acid, Certified Reference Material, Tracecert(r)
77. Inchi=1/c5h8o4/c6-4(7)2-1-3-5(8)9/h1-3h2,(h,6,7)(h,8,9
| Molecular Weight | 132.11 g/mol |
|---|---|
| Molecular Formula | C5H8O4 |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 74.6 |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 104 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EXPTL USE: GLUTARIC ACID HAD IN VITRO VIRUCIDAL ACTIVITY AGAINST LARGE NUMBER OF VIRUSES SUCH AS RHINOVIRUS & HERPES.
GUY DD; COMPOSITION CONTAINING GLUTARIC ACID AS A VIRUCIDAL AGENT; EUR PAT APPL PATENT NUMBER 8121 02/20/80 (STERLING DRUG IND)
MEDICATION (VET): GLUTARIC ACID & P-AMINOBENZOIC ACID BLOCKED NET FLUID SECRETION CAUSED BY CHOLERA TOXIN OR THE HEAT-STABLE ENTEROTOXIN OF ESCHERICHIA COLI. THE TISSUE EXAMINED WAS LIGATED JEJUNAL LOOPS IN WEANLING PIGS.
FORSYTH GW ET AL; ORGANIC ACID PROTON DONORS DECREASE INTESTINAL SECRETION CAUSED BY ENTEROTOXINS; AM J PHYSIOL 241(3) G227 (1981)
AGENT IN ANIMAL DIABETES & BIOCHEMICAL RESEARCH
SRI
RAT LIVER MITOCHONDRIA METABOLIZED GLUTARATE VERY SLOWLY COMPARED WITH GLUTARYL-COENZYME A (COA). GLUTARYL-COA DEHYDROGENASE, WHICH CATALYZES THE STOICHIOMETRIC CONVERSION OF GLUTARYL-COA TO 1 MOLE EACH OF CARBON DIOXIDE AND CROTONYL-COA OR ITS INTERMEDIATE METABOLITE, WAS PURIFIED APPROX 44- AND 100-FOLD FROM BOVINE LIVER AND KIDNEY MITOCHONDRIA, RESPECTIVELY. THE KM FOR GLUTARYL-COA WAS 3.3 MUM.
BESRAT A ET AL; MAMMALIAN METABOLISM OF GLUTARIC ACID; J BIOL CHEM 244(6) 1461 (1969)
