



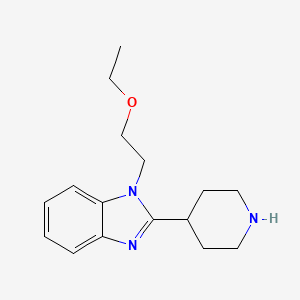

1. 110963-63-8
2. 1-(2-ethoxyethyl)-2-piperidin-4-ylbenzimidazole
3. 1-(2-ethoxy-ethyl)-2-piperidin-4-yl-1h-benzimidazole
4. 1-(2-ethoxyethyl)-2-(4-piperidinyl)-1h-benzimidazole
5. 1-(2-ethoxyethyl)-2-(piperidin-4-yl)-1h-benzo[d]imidazole Oxalate
6. Schembl991263
7. Amy23147
8. Bcp08770
9. Zinc34160220
10. Akos027383994
11. Ac-29251
12. Db-060051
13. Ft-0660492
14. 1-(2-ethoxyethyl)-2-piperidin-4-yl-1h-benzimidazole
15. 1-(2-ethoxyethyl)-2-piperidin-4-yl-1h-benzoimidazole
16. 1-(2-ethoxyethyl)-2-(piperidin-4-yl)-1h-1,3-benzodiazole;1-(2-ethoxyethyl)-2-(piperidin-4-yl)-1h-benzo[d]imidazole
| Molecular Weight | 273.37 g/mol |
|---|---|
| Molecular Formula | C16H23N3O |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 273.184112366 g/mol |
| Monoisotopic Mass | 273.184112366 g/mol |
| Topological Polar Surface Area | 39.1 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 293 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




