
1. Arixtra
2. Fondaparinux
3. Quixidar
1. 114870-03-0
2. Arixtra
3. Quixidar
4. Fondaparin Sodium
5. Sr-90107a
6. Org 31540
7. Sr 90107a
8. Fondaparinux Sodium For Assay
9. Org-31540
10. Fondaparinux Sodium Identification
11. X0q6n9usoz
12. Ic-851589
13. Xantidar
14. Arixtra (tn)
15. Unii-x0q6n9usoz
16. Fondaparinux Sodium [usan]
17. Fondaparinux Sodium [usan:inn:ban]
18. Gsk-576428
19. Ic 85158
20. Sr-90107
21. Chembl1200644
22. Fondaparinux Sodium [mi]
23. Fondaparinux Sodium [inn]
24. Fondaparinux Sodium [jan]
25. Dtxsid501027612
26. Fondaparinux Sodium [mart.]
27. Fondaparinux Sodium (jan/usp/inn)
28. Fondaparinux Sodium [usp-rs]
29. Fondaparinux Sodium [who-dd]
30. Natural Heparin Pentasaccharide Sodium
31. Akos005146286
32. Fondaparinux Sodium [ema Epar]
33. Ccg-270693
34. Fondaparinux Sodium [orange Book]
35. As-80003
36. Fondaparinux Sodium [usp Monograph]
37. C71476
38. D01844
39. A803253
40. Q421218
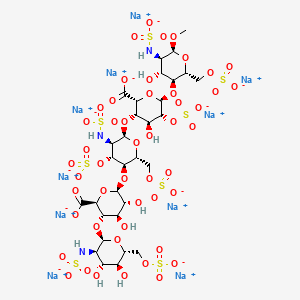
41. Alpha-d-glucopyranoside, Methyl O-2-deoxy-6-o-sulfo-2-(sulfoamino)-alpha-d-glucopyranosyl-(1-4)-o-beta-d-glucopyranuronosyl(1-4)-o-2-deoxy-3,6-di-o-sulfo-2-(sulfoamino)-alpha-d-glucopyranosyl-(1-4)-o-2-o-sulfo-alpha-l-idopyranuronosyl-(1-4)-2-deoxy-2-(sulfoamino)-, 6-(hydrogen Sulfate), Decasodium Salt
42. Decasodium 6-[6-[2-carboxylato-4-hydroxy-6-[4-hydroxy-6-methoxy-5-(sulfonatoamino)-2-(sulfonatooxymethyl)tetrahydropyran-3-yl]oxy-5-sulfonatooxy-tetrahydropyran-3-yl]oxy-5-(sulfonatoamino)-4-sulfonatooxy-2-(sulfonatooxymethyl)tetrahydropyran-3-yl]oxy-3-[4;fondaparinux Sodium
43. Methly O-2-deoxy-6-o-sulfo-2-(sulfoamino)-alpha-d-glucopyranosyl-(1-4)-o-beta-d-glucopyranuronosyl-(1-4)-o-2-deoxy-3,6-di-o-sulfo-2-(sulfoamino)-alpha-d-glucopyranosyl-(1-4)-o-2-o-sulfo-alpha-l-idopyranuronosyl-(1-4)-2-deoxy-6-o-sulfo-2-(sulfoamino)-alpha-d-glucopyranoside, Decasodium Salt
| Molecular Weight | 1728.1 g/mol |
|---|---|
| Molecular Formula | C31H43N3Na10O49S8 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 52 |
| Rotatable Bond Count | 20 |
| Exact Mass | 1726.7707769 g/mol |
| Monoisotopic Mass | 1726.7707769 g/mol |
| Topological Polar Surface Area | 901 Ų |
| Heavy Atom Count | 101 |
| Formal Charge | 0 |
| Complexity | 3330 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 25 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 11 |
* 1. 5-mg/0. 3-ml and 2. 5-mg/0. 5-ml solution for injection:
Prevention of venous thromboembolic events (VTE) in adults undergoing major orthopaedic surgery of the lower limbs such as hip fracture, major knee surgery or hip-replacement surgery.
Prevention of VTE in adults undergoing abdominal surgery who are judged to be at high risk of thromboembolic complications, such as patients undergoing abdominal cancer surgery.
Prevention of VTE in adult medical patients who are judged to be at high risk for VTE and who are immobilised due to acute illness such as cardiac insufficiency and / or acute respiratory disorders, and / or acute infectious or inflammatory disease.
Treatment of adults with acute symptomatic spontaneous superficial-vein thrombosis of the lower limbs without concomitant deep-vein thrombosis.
* 2. 5-mg/0. 5-ml solution for injection:
Treatment of unstable angina or non-ST-segment-elevation myocardial infarction (UA/NSTEMI) in adult patients for whom urgent (< 120 mins) invasive management (PCI) is not indicated.
infarction (STEMI) in adult patients who are managed with thrombolytics or who initially are to receive no other form of reperfusion therapy.
* 5-mg/0. 4-ml, 7. 5-mg/0. 6-ml and 10-mg/0. 8-ml solution for injection:
Treatment of adults with acute deep-vein thrombosis (DVT) and treatment of acute pulmonary embolism (PE), except in haemodynamically unstable patients or patients who require thrombolysis or pulmonary embolectomy.
1. 5 mg/0. 3 ml and 2. 5 mg/0. 5 ml, solution for injection:
Prevention of Venous Thromboembolic Events (VTE) in patients undergoing major orthopaedic surgery of the lower limbs such as hip fracture, major knee surgery or hip replacement surgery.
Prevention of Venous Thromboembolic Events (VTE) in patients undergoing abdominal surgery who are judged to be at high risk of thromboembolic complications, such as patients undergoing abdominal cancer surgery (see section 5. 1).
Prevention of Venous Thromboembolic Events (VTE) in medical patients who are judged to be at high risk for VTE and who are immobilised due to acute illness such as cardiac insufficiency and/or acute respiratory disorders, and/or acute infectious or inflammatory disease.
2. 5 mg/0. 5 ml, solution for injection:
Treatment of unstable angina or non-ST segment elevation myocardial infarction (UA/NSTEMI) in patients for whom urgent (< 120 mins) invasive management (PCI) is not indicated (see sections 4. 4 and 5. 1).
Treatment of ST segment elevation myocardial infarction (STEMI) in patients who are managed with thrombolytics or who initially are to receive no other form of reperfusion therapy.
5 mg/0. 4 ml, 7. 5 mg/0. 6 ml and 10 mg/0. 8 ml solution for injection:
Treatment of acute Deep Vein Thrombosis (DVT) and treatment of acute Pulmonary Embolism (PE), except in haemodynamically unstable patients or patients who require thrombolysis or pulmonary embolectomy.
Factor Xa Inhibitors
Endogenous factors and drugs that inhibit or block the activity of FACTOR XA. (See all compounds classified as Factor Xa Inhibitors.)
B01AX05
B01AX05

