



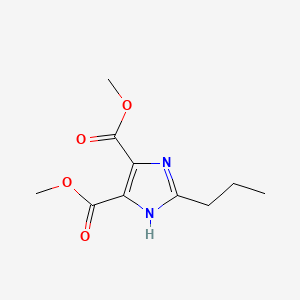

1. 124750-59-0
2. 2-propyl-1h-imidazole-4,5-dicarboxylic Acid Dimethyl Ester
3. 1h-imidazole-4,5-dicarboxylic Acid, 2-propyl-, Dimethyl Ester
4. Schembl784
5. Dtxsid80570231
6. 1h-imidazole-4,5-dicarboxylic Acid, 2-propyl-, 4,5-dimethyl Ester
7. Mfcd09743546
8. Zinc21297655
9. Akos015914937
10. 2-n-propyl-4,5-dicarbomethoxyimidazole
11. 4,5-dicarbomethoxy-2-n-propylimidazole
12. As-71868
13. 2-propyl-4,5-bis(methoxycarbonyl)-imidazole
14. Cs-0152408
15. Ft-0657730
16. E76022
17. 750p590
18. A805290
19. J-520360
20. 2-propyl-1h-imidazole-4,5-dicarboxylicaciddimethylester
21. 1h-imidazole-4,5-dicarboxylic Acid,2-propyl-,dimethyl Ester
22. 4,5-dimethyl 2-propyl-1h-imidazole-4,5-dicarboxylate
| Molecular Weight | 226.23 g/mol |
|---|---|
| Molecular Formula | C10H14N2O4 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 226.09535693 g/mol |
| Monoisotopic Mass | 226.09535693 g/mol |
| Topological Polar Surface Area | 81.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





