

X



1. 125092-42-4
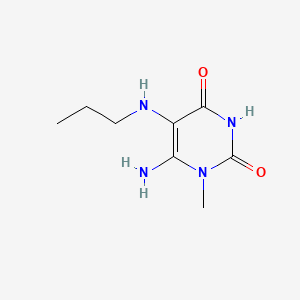
2. 6-amino-1-methyl-5-(propylamino)uracil
3. 6-amino-1-methyl-5-(propylamino)pyrimidine-2,4-dione
4. 6-amino-5-propylamino-1-methyluracil
5. 2,4(1h,3h)-pyrimidinedione,6-amino-1-methyl-5-(propylamino)-
6. Dtxsid70559939
7. Mfcd01074825
8. Zinc37486424
9. Akos008962125
10. Sb55600
11. As-71163
12. Cs-0256311
13. Ft-0620928
14. T70174
15. 6-amino-1-methyl-5-(propylamino)-1,2,3,4-tetrahydropyrimidine-2,4-dione
| Molecular Weight | 198.22 g/mol |
|---|---|
| Molecular Formula | C8H14N4O2 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 198.11167570 g/mol |
| Monoisotopic Mass | 198.11167570 g/mol |
| Topological Polar Surface Area | 87.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 298 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




