
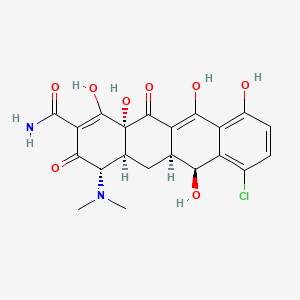

1. Declomycin
2. Demeclocycline Hydrochloride
3. Demeclocycline Monohydrochloride
4. Demeclocycline, 4-epimer
5. Demeclocycline, Calcium (1:1) Salt
6. Demeclocycline, Calcium (1:2) Salt
7. Demethylchlortetracycline
8. Hydrochloride, Demeclocycline
9. Ldermycine
10. Ledermycin
11. Monohydrochloride, Demeclocycline
1. Demethylchlortetracycline
2. Dmct
3. 127-33-3
4. Ledermycin
5. Demethylchlortetracyclin
6. 6-demethyl-7-chlorotetracycline
7. 7-chloro-6-demethyltetracycline
8. Demeclociclina
9. Demeclocyclinum
10. Demethylchlorotetracycline
11. Demethylchlortetracyclinum
12. 6-demethylchlorotetracycline
13. Tri-demethylchlortetracycline
14. 6-demethylchlortetracycline
15. Dmctc
16. Demeclocyclinum [inn-latin]
17. Demeclociclina [inn-spanish]
18. Novotriclina
19. Demeclor
20. Demetraclin
21. Diuciclin
22. Mexocine
23. Perciclina
24. Sumaclina
25. Deganol
26. Dmct (antibiotic)
27. Demethylchlortetracycline Base
28. 5r5w9ici6o
29. 6-demethyl-7-chlortetracycline
30. Chebi:4392
31. 127-33-3 (free Base)
32. Chlortetracycline, 6-demethyl-
33. (4s,4as,5as,6s,12ar)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
34. (4s,4as,5as,6s,12as)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
35. 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide
36. Tetracycline, 7-chloro-6-demethyl-
37. Rp 10192
38. [4s-(4alpha,4aalpha,5aalpha,6beta,12aalpha)]-7-chloro-4-(dimethylamino)1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide
39. Demeclocycline [usan:ban]
40. Demethylchlortetracycline (jan)
41. Demethylchlortetracycline [jan]
42. 6-demetil-7-clorotetraciclina
43. Hsdb 3051
44. Methylchlorotetracycline
45. Einecs 204-834-8
46. Unii-5r5w9ici6o
47. 6-demetil-7-clorotetraciclina [italian]
48. Demeclocycline [usp:inn:ban]
49. Demeclocycline (usp)
50. Spectrum_000896
51. Prestwick0_000753
52. Prestwick1_000753
53. Prestwick2_000753
54. Prestwick3_000753
55. Spectrum2_001132
56. Spectrum3_000378
57. Spectrum4_000313
58. Spectrum5_000831
59. Demeclocycline [mi]
60. Schembl3252
61. Chembl1591
62. Demeclocycline [inn]
63. Lopac0_000421
64. Bspbio_000766
65. Bspbio_002135
66. Demeclocycline [hsdb]
67. Kbiogr_000926
68. Kbioss_001376
69. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-, (4s-(4alpha,4aalpha,5aalpha,6beta,12aalpha))-
70. Demeclocycline [vandf]
71. Divk1c_000863
72. Schembl516084
73. Spbio_001023
74. Spbio_002705
75. Demeclocycline [mart.]
76. Bpbio1_000844
77. Demeclocycline [who-dd]
78. Dtxsid1022893
79. Schembl13169106
80. Schembl17710674
81. Gtpl10905
82. Kbio1_000863
83. Kbio2_001376
84. Kbio2_003944
85. Kbio2_006512
86. Kbio3_001355
87. Ninds_000863
88. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-, (4s-(4.alpha.,4a.alpha.,5a.alpha.,6.beta.,12a.alpha.))-
89. Rkl10089
90. Demeclocycline [usp Monograph]
91. Zinc100036924
92. Ccg-204513
93. Db00618
94. Sdccgsbi-0050406.p005
95. Idi1_000863
96. Smp1_000091
97. 1,3,5-benzenetrisulfonylchloride
98. Ncgc00162149-02
99. Ncgc00162149-03
100. Ncgc00162149-04
101. Ncgc00162149-06
102. Ncgc00162149-10
103. Ncgc00162149-14
104. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-, (4s,4as,5as,6s,12as)-
105. Sbi-0050406.p004
106. Hy-121268
107. Cs-0081339
108. D03680
109. Ab00053449_04
110. Sr-01000000066
111. J-005483
112. Q2736402
113. Sr-01000000066-4
114. Chlortetracycline Hydrochloride Impurity B [ep Impurity]
115. (4s,4as,5as,6s,12as)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
116. 7-chloro-4-dimethylamino-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic Acid Amide
| Molecular Weight | 464.9 g/mol |
|---|---|
| Molecular Formula | C21H21ClN2O8 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 464.0986433 g/mol |
| Monoisotopic Mass | 464.0986433 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 961 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Tetracycline
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Tetracyclines are especially useful in diseases caused by rickettsiae, mycoplasmas, & chlamydiae. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1243
...THEY ARE OF PROVEN VALUE.../AGAINST/ ROCKY MOUNTAIN SPOTTED FEVER, MURINE TYPHUS, RECRUDESCENT EPIDEMIC TYPHUS, SCRUB TYPHUS, Q FEVER, LYMPHOGRANULOMA VENEREUM, PSITTACOSIS, TULAREMIA, BRUCELLOSIS, GONORRHEA, CERTAIN URINARY TRACT INFECTIONS, OCULAR INFECTIONS, GRANULOMA INGUINALE, CHANCROID, SYPHILIS... /TETRACYCLINES/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1241
...THEY ARE OF PROVEN VALUE.../AGAINST/ DISEASE DUE TO BACTEROIDES & CLOSTRIDIUM. /TETRACYCLINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1191
For more Therapeutic Uses (Complete) data for DEMECLOCYCLINE (8 total), please visit the HSDB record page.
... /Tetracyclines/ should not be given to pregnant patients; they should not be employed for treatment of common infections in children under the age of 8 yr; & unused supplies of these antibiotics should be discarded. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1246
Tetracyclines may aggravate uremia in patients with renal disease by inhibiting protein synthesis & provoking a catabolic effect. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1245
Tetracyclines are incompletely absorbed from the GI tract, such that high concns are reached in the bowel, & therefore the enteric flora is markedly altered. Many aerobic & anaerobic coliform microorganisms & gram-positive spore-forming bacteria are sensitive & may be suppressed markedly during long-term tetracycline regimens before resistant strains reappear. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1241
Because of the emergence of resistance, the tetracyclines are no longer indicated for infections caused by staphylococci, streptococci, or meningococci. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1244
For more Drug Warnings (Complete) data for DEMECLOCYCLINE (19 total), please visit the HSDB record page.
2 TO 3. 2= SLIGHTLY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-15 G/KG, BETWEEN 1 PINT & 1 QT FOR 70 KG PERSON (150 LB). 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-172
Used primarily to treat Lyme disease, acne, and bronchitis. Also indicated (but rarely used) to treat urinary tract infections, gum disease, malaria, and other bacterial infections such as gonorrhea and chlamydia. One of its other registered uses is the treatment of hyponatremia (low blood sodium concentration) due to the syndrome of inappropriate antidiuretic hormone (SIADH) where fluid restriction alone has been ineffective.
Demeclocycline is a tetracycline antibiotic active against the following microorganisms: Rickettsiae (Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsial pox, tick fevers), Mycoplasma pneumoniae (PPLO, Eaton agent), agents of psittacosis and ornithosis, agents of lymphogranulomavenereum and granuloma inguinale, the spirochetal agent of relapsing fever (Borrelia recurrentis), Haemophilus ducreyi (chancroid), Yersinia pestis, Pasteurella pestis and Pasteurella tularensis, Bartonella bacilliformis, Bacteroides species, Vibrio comma and Vibrio fetus, and Brucella species (in conjunction with streptomycin). Demeclocycline inhibits cell growth by inhibiting translation. Demeclocycline is lipophilic and can easily pass through the cell membrane or passively diffuses through porin channels in the bacterial membrane. Demeclocycline is not a direct bactericidal agent; rather, it is a bacteriostatic drug that impairs bacterial growth. Because it is excreted more slowly than tetracycline, it maintains effective blood levels for longer periods of time.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AA - Tetracycline and derivatives
D06AA01 - Demeclocycline
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA01 - Demeclocycline
Absorption
Tetracyclines are readily absorbed.
Route of Elimination
Demeclocycline hydrochloride, like other tetracyclines, is concentrated in the liver and excreted into the bile where it is found in much higher concentrations than in the blood. Following a single 150 mg dose of demeclocycline hydrochloride in normal volunteers, 44% (n = 8) was excreted in urine and 13% and 46%, respectively, were excreted in feces in two patients within 96 hours as active drug.
Clearance
Renal cl=35 mL/min/1.73 m2
Tetracyclines are deposited in the skeleton during gestation & throughout childhood. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1245
Tetracyclines distribute widely throughout the body & into tissues & secretions, including the urine & prostate. They accumlate in the reticuloendothelial cells of the liver, spleen, & bone marrow, & in bone, dentine, & the enamel of unerupted teeth. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1242
Tetracyclines cross the placenta & enter the fetal circulation & amniotic fluid. Concn of tetracycline in umbilical-cord plasma reach 60%, & in amniotic fluid 20%, of those in the circulation of the mother. Relatively high concns of these drugs also are found in breast milk. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1242
AFTER ORAL ADMIN OF DEMETHYLCHLORTETRACYCLINE (FREE BASE & HCL SALT) TO RATS, PLASMA LEVELS FROM EACH WERE ESSENTIALLY IDENTICAL.
MIYAZAKI S ET AL; COMPARISON OF BIOAVAILABILITY OF FREE BASES & HCL SALTS OF CHLORTETRACYCLINE, DEMETHYLCHLORTETRACYCLINE & METHACYCLINE; CHEM PHARM BULL 23 (9): 2151 (1975)
The newer tetracyclines are defined as those tetracyclines available in the United States but not approved for veterinary use. These include demeclocycline, methacycline, doxycycline, & minocycline. Of these, doxycycline & minocycline appear to offer advantages that would render them useful in certain situations in veterinary medicine. Their major advantage lies in their greater lipid solubility relative to other tetracyclines. This characteristic probably accounts for their enhanced antimicrobial effectiveness for some organisms, more efficient absorption after oral admin, & enhanced distribution in the body. The principal excretory organ for doxycycline is the intestine, where the drug diffuses through the intestinal mucosa into the intestinal tract. This unique characteristic makes this drug useful in cases of preexisting renal dysfunction & may render this drug superior to other tetracyclines in the treatment of intestinal infections. Doxycycline is used in other countries for respiratory tract & intestinal tract diseases of poultry. The usefulness of doxycycline & minocycline in food-producing animals may be limited because of persistent drug residues. Minocycline has, in large doses, been used with streptomycin in the elimination of the carrier state of canine brucellosis. The superiority of doxycycline & minocycline, relative to other tetracyclines, in their distribution to areas of the body such as the eye, brain, cerebrospinal fluid, & prostate gland suggests that trials of their efficacy in tetracycline-sensitive infections of these areas are indicated. Pharmacokinetic studies designed to determine optimal dosage schedules have not been made for domestic animals. These determinations are necessary to evaluate most effectively the usefulness of the newer tetracyclines in veterinary medicine.
PMID:7216873 Aronson AL; J Am Vet Med Assoc 176 (10 Spec No): 1061-1068 (1980)
Hepatic
10-17 hours
IN PLASMA, IT IS PROTEIN-BOUND 41 TO 90%. ITS T/2 IS ABOUT 12 HR. /HCL/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1139
Demeclocycline inhibits cell growth by inhibiting translation. It binds (reversibly) to the 30S and 50S ribosomal subunit and prevents the amino-acyl tRNA from binding to the A site of the ribosome, which impairs protein synthesis by bacteria. The binding is reversible in nature. The use in SIADH actually relies on a side-effect of tetracycline antibiotics; many may cause diabetes insipidus (dehydration due to the inability to concentrate urine). It is not completely understood why demeclocycline impairs the action of antidiuretic hormone, but it is thought that it blocks the binding of the hormone to its receptor.
Tetracyclines inhibit bacterial protein synthesis by binding to the 30 S bacterial ribosome & preventing access of aminoacyl tRNA to the acceptor (A) site on the mRNA-ribosome complex. They enter gram-negative bacteria by passive diffusion through the hydrophilic channels, formed by the porin proteins of the outer cell membrane, & active transport by an energy-dependent system that pumps all tetracyclines across cytoplasmic membrane. Although permeation of these drugs into gram-positive bacteria is less well understood it also is energy requiring. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1241
...INHIBITORY EFFECTS OF TETRACYCLINES CAN BE REVERSED BY WASHING. THEREFORE, IT IS PROBABLE THAT REVERSIBLY BOUND ANTIBIOTIC IS RESPONSIBLE FOR ANTIBACTERIAL ACTION. /TETRACYCLINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1185
... Drugs to antagonize /antidiuretic hormone/, ADH, action can be used /to treat the syndrome of inappropriate antidiuretic hormone secretion/. These include a loop diuretic, demeclocycline ... . ... Demeclocycline & lithium directly impair the response to ADH at the collecting tubule, inducing nephrogenic diabetes insipidus. Demeclocycline usually is better tolerated than lithium.
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 28-10
BUILDING BLOCK

