

1. 13529-31-2
2. (2b,3a,16b,17b)-2,16-bispiperidino-3,17-diacetoxy-5-androstane
3. N738ko204j
4. Vecuronium Bromide Related Compound A
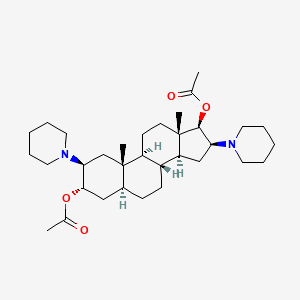
5. [(2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-17-acetyloxy-10,13-dimethyl-2,16-di(piperidin-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl] Acetate
6. Androstane-3,17-diol, 2,16-di-1-piperidinyl-, 3,17-diacetate, (2beta,3alpha,5alpha,16beta,17beta)-
7. Androstane-3,17-diol,2,16-di-1-piperidinyl-, 3,17-diacetate, (2b,3a,5a,16b,17b)-
8. Unii-n738ko204j
9. Pancuronium Bromide Specified Impurity E [ep]
10. Schembl5795960
11. Dtxsid201133509
12. 2beta,16beta-dipiperidino-5alpha-androstane-3alpha,17beta-diol Diacetate
13. Zinc118912865
14. (2beta,3alpha,5alpha,16beta,17beta)-2,16-bispiperidino-3,17-diacetoxy-5-androstane
15. D88040
16. 529d312
17. Pancuronium Bromide Impurity E [ep Impurity]
18. Vecuronium Bromide Impurity A [ep Impurity]
19. Q27284650
20. Vecuronium Bromide Related Compound A [usp-rs]
21. Vecuronium Bromide Related Compound A [usp Impurity]
22. 2b,3a,16b,17b)-2,16-bispiperidino-3,17-diacetoxy-5-androstane
23. (2?,3a,5a,16?,17?)-androstane-3,17-diol-2,16-di-1-piperidinyldiacetic Acid Ester
24. (2beta, 3alpha, 16beta, 17beta)-3,17-diacetoxy-2,16-dipiperidino-5alpha-androstane
25. 2.beta.,16.beta.-dipiperidino-5.alpha.-androstane-3.alpha.,17.beta.-diol Diacetate
26. (2.beta.,3.alpha.,5.alpha.,16.beta.,17.beta.)-2,16-bispiperidino-3,17-diacetoxy-5-androstane
27. 2.beta.,16.beta.-bis(piperidin-1-yl)-5.alpha.-androstane-3.alpha.,17.beta.-diyl Diacetate
28. Androstane-3,17-diol, 2,16-di-1-piperidinyl-, 3,17-diacetate, (2.beta.,3.alpha.,5.alpha.,16.beta.,17.beta.)-
| Molecular Weight | 542.8 g/mol |
|---|---|
| Molecular Formula | C33H54N2O4 |
| XLogP3 | 6.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 542.40835821 g/mol |
| Monoisotopic Mass | 542.40835821 g/mol |
| Topological Polar Surface Area | 59.1 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 913 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
BUILDING BLOCK

