

1. Gs-5816
1. 1377049-84-7
2. Gs-5816
3. Gs5816
4. Kcu0c7rs7z
5. Gs 5816
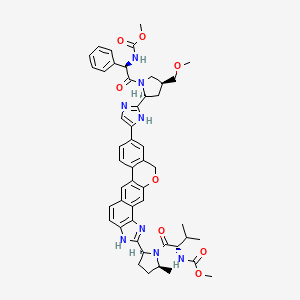
6. Methyl ((r)-2-((2s,4s)-2-(5-(2-((2s,5s)-1-((methoxycarbonyl)-l-valyl)-5-methylpyrrolidin-2-yl)-1,11-dihydroisochromeno[4',3':6,7]naphtho[1,2-d]imidazol-9-yl)-1h-imidazol-2-yl)-4-(methoxymethyl)pyrrolidin-1-yl)-2-oxo-1-phenylethyl)carbamate
7. Methyl [(2s)-1-[(2s,5s)-2-[9-[2-[(2s,4s)-1-[(2r)-2-[(methoxycarbonyl)amino]-2-phenylacetyl]-4-(methoxymethyl)pyrrolidin-2-yl]-1h-imidazol-5-yl]-1,11-dihydroisochromeno[4',3':6,7]naphtho[1,2-d]imidazol-2-yl]-5-methylpyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate
8. Methyl N-[(1r)-2-[(2s,4s)-2-[5-[6-[(2s,5s)-1-[(2s)-2-(methoxycarbonylamino)-3-methylbutanoyl]-5-methylpyrrolidin-2-yl]-21-oxa-5,7-diazapentacyclo[11.8.0.03,11.04,8.014,19]henicosa-1(13),2,4(8),5,9,11,14(19),15,17-nonaen-17-yl]-1h-imidazol-2-yl]-4-(methoxymethyl)pyrrolidin-1-yl]-2-oxo-1-phenylethyl]carbamate
9. Unii-kcu0c7rs7z
10. Velpatasvir [usan:inn]
11. Verpatasvir
12. Methyl ((2s)-1-((2s,5s)-2-(9-(2-((2s,4s)-1-((2r)-2-((methoxycarbonyl)amino)-2-phenylacetyl)-4-(methoxymethyl)pyrrolidin-2-yl)-1h-imidazol-5-yl)-1,11-dihydroisochromeno[4',3':6,7]naphtho[1,2-d]imidazol-2-yl)-5-methylpyrrolidin-1-yl)-3-methyl-1-oxobutan-2-yl)carbamate
13. Methyl {(2s)-1-[(2s,5s)-2-(9-{2-[(2s,4s)-1-{(2r)-2-[(methoxycarbonyl)amino]-2-phenylacetyl}-4-(methoxymethyl)pyrrolidin-2-yl]-1h-imidazol-5-yl}-1,11-dihydroisochromeno[4',3':6,7]naphtho[1,2-d]imidazol-2-yl)-5-methylpyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl}carbamate
14. Velpatasvir [inn]
15. Velpatasvir(gs5816)
16. Velpatasvir [mi]
17. Velpatasvir [jan]
18. Velpatasvir; Gs-5816
19. Velpatasvir [usan]
20. Velpatasvir [who-dd]
21. Velpatasvir (jan/usan/inn)
22. Schembl8756902
23. Chembl3545062
24. Schembl19236068
25. Gtpl11269
26. Amy4211
27. Dtxsid70722565
28. Velpatasvir [orange Book]
29. Chebi:133009
30. Epclusa (sofosbuvir + Velpatasvir)
31. Vosevi Component Velpatasvir
32. Bcp13813
33. Epclusa Component Velpatasvir
34. Ex-a2320
35. Bdbm50521720
36. Mfcd28411371
37. S3724
38. Velpatasvir Component Of Vosevi
39. Zinc203686879
40. Ccg-270553
41. Cs-5977
42. Db11613
43. Velpatasvir Component Of Epclusa
44. Ncgc00522503-01
45. Ncgc00522503-02
46. Ac-28744
47. As-35237
48. Hy-12530
49. Ft-0700879
50. J3.631.686a
51. D10806
52. Gs-5816;gs5816;gs 5816
53. Vosevi (sofosbuvir + Velpatasvir + Voxilaprevi)
54. A886410
55. Q25018296
56. Carbamic Acid, N-[(1r)-2-[(2s,4s)-2-[5-[1,11-dihydro-2-[(2s,5s)-1-[(2s)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxobutyl]-5-methyl-2-pyrrolidinyl][2]benzopyrano[4,3:6,7]naphth[1,2-d]imidazol-9-yl]-1h-imidazol-2-yl]-4-(methoxymethyl)-1-pyrrolidinyl]-2-oxo-1-phenylethyl]-, Methyl Ester
57. Methyl {(2s)-1-[(2s,5s)-2-(9-{2-[(2s,4s)-1-{(2r)-2-[(methoxycarbonyl)amino]-2-phenylacetyl}-4-(methoxymethyl)pyrrolidin-2-yl]-1h-imidazol-4-yl}-1,11-dihydro[2]benzopyrano[4',3':6,7]naphtho[1,2-d]imidazol-2-yl)-5-methylpyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl}carbamate
58. Methyl N-[(1r)-2-[(2s,4s)-2-(5-{6-[(2s,5s)-1-[(2s)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl]-5-methylpyrrolidin-2-yl]-21-oxa-5,7-diazapentacyclo[11.8.0.0?,??.0?,?.0??,??]henicosa-1,3,6,8,10,12,14,16,18-nonaen-17-yl}-1h-imidazol-2-yl)-4-(methoxymethyl)pyrrolidin-1-yl]-2-oxo-1-phenylethyl]carbamate
59. Methyl N-[(1r)-2-[(2s,4s)-2-(5-{6-[(2s,5s)-1-[(2s)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl]-5-methylpyrrolidin-2-yl]-21-oxa-5,7-diazapentacyclo[11.8.0.03,11.0?,?.01?,1?]henicosa-1,3,6,8,10,12,14,16,18-nonaen-17-yl}-1h-imidazol-2-yl)-4-(methoxymethyl)pyrrolidin-1-yl]-2-oxo-1-phenylethyl]carbamate
60. Methyl{(2s)-1-[(2s,5s)-2-(9-{2-[(2s,4s)-1-{(2r)-2-[(methoxycarbonyl)amino]-2-phenylacetyl}-4-(methoxymethyl)pyrrolidin-2-yl]-1h-imidazol-5-yl}-1,11-dihydroisochromeno[4',3':6,7]naphtho[1,2-d]imidazol-2-yl)-5-methylpyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl}carbamate
| Molecular Weight | 883.0 g/mol |
|---|---|
| Molecular Formula | C49H54N8O8 |
| XLogP3 | 6.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 13 |
| Exact Mass | 882.40646071 g/mol |
| Monoisotopic Mass | 882.40646071 g/mol |
| Topological Polar Surface Area | 193 Ų |
| Heavy Atom Count | 65 |
| Formal Charge | 0 |
| Complexity | 1690 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | EPCLUSA |
| Active Ingredient | SOFOSBUVIR; VELPATASVIR |
| Company | GILEAD SCIENCES INC (Application Number: N208341. Patents: 7964580, 8334270, 8575135, 8580765, 8618076, 8633309, 8735372, 8889159, 8921341, 8940718, 9085573, 9284342, 9757406) |
| 2 of 2 | |
|---|---|
| Drug Name | VOSEVI |
| Active Ingredient | SOFOSBUVIR; VELPATASVIR; VOXILAPREVIR |
| Company | GILEAD SCIENCES INC (Application Number: N209195. Patents: 7964580, 8334270, 8575135, 8580765, 8618076, 8633309, 8735372, 8889159, 8921341, 8940718, 9085573, 9284342, 9296782, 9585906, 9868745) |
Velpatasvir is used in combination therapy with other antiviral medications to treat chronic hepatitis C virus (HCV) infected patients with HCV genoptypes 1-6, and to treat HCV and HIV co-infected patients. Depending on the level of cirrhosis or decompensation, combination therapy can also include therapy with [DB00811]. When used in combination with [DB08934] as the combination product Epclusa, Velpatasvir is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis, or in combination with [DB00811] if associated with decompensated cirrhosis.
FDA Label
Velpatasvir is a small molecule direct-acting antiviral used in the treatment of hepatitis C in combination with sofosbuvir. Velpatasvir prevents viral replication by inhibiting non-structural protein 5A (NS5A). At a dose 5 times the recommended dose, velpatasvir does not prolong QTc interval to any clinically relevant extent.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Absorption
Oral bioavailability of 25-30%.
Route of Elimination
94% excreted in feces with 77% as parent compound. 0.4% excreted in urine.
Volume of Distribution
1.4-1.6 L/kg.
Clearance
Estimated 0.12 L/h/kg [A19175.
Some metabolism by CYP2B6, CYP2C8, and CYP3A4.
15h.
Velpatasvir's mechanism of action is likely similar to other selective NS5A inhibitors which bind domain I of NS5A consisting of amino acids 33-202. NS5A inhibitors compete with RNA for binding at this site. It is also thought that NS5A inhibitors bind the target during its action in replication when the binding site is exposed. Inhibition of NS5A is also known to produce redistribution of the protein to lipid droplets. The exact role of NS5A in RNA replication is not yet understood although it is known to be an important component.
BUILDING BLOCK


