



1. 147816-24-8
2. Cefcapene Pivoxil Hydrochloride Monohydrate
3. Cefcapene Pivoxil Hydrochloride Hydrate
4. V16a6ayi9h
5. S 1108
6. Flomox
7. Cefcapene Pivoxil (hydrochloride Hydrate)
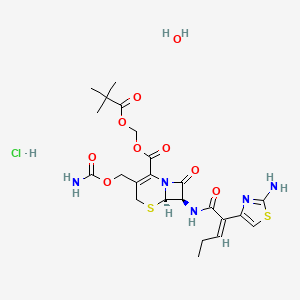
8. 2,2-dimethylpropanoyloxymethyl (6r,7r)-7-[[(z)-2-(2-amino-1,3-thiazol-4-yl)pent-2-enoyl]amino]-3-(carbamoyloxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;hydrate;hydrochloride
9. S-1108
10. Unii-v16a6ayi9h
11. Flomox (tn)
12. Cfpn-pi
13. Cefcapene Pivoxil Hcl H2o
14. Cefcapenepivoxil Hydrochloride
15. Schembl4755909
16. Chembl4303585
17. J01dd17
18. Bcp12048
19. Ex-a4006
20. Mfcd01682041
21. S4844
22. Akos025310133
23. Ccg-270257
24. Cs-w020762
25. Hy-w040022
26. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-((2-(2-amino-4-thiazolyl)-1-oxo-2-pentenyl)amino)-8-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester,(6r-(6-alpha,7-beta(z)))-, Monohydrochloride, Hydrate
27. As-65791
28. Cefcapene Pivoxil Hydrochloride Hydrate (jp17)
29. D01680
30. 816c248
31. Cefcapene Pivoxil Hydrochloride Hydrate [jan]
32. Cefcapene Pivoxil Hydrochloride Hydrate [mi]
33. J-700157
34. Cefcapene Pivoxil Monohydrochloride Monohydrate
35. Q27291405
36. Cefcapene Pivoxil Hydrochloride Hydrate [who-dd]
37. (6r,7r)-(pivaloyloxy)methyl 7-((z)-2-(2-aminothiazol-4-yl)pent-2-enamido)-3-((carbamoyloxy)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate Hydrochloride Hydrate
38. (6r,7r)-(pivaloyloxy)methyl7-((z)-2-(2-aminothiazol-4-yl)pent-2-enamido)-3-((carbamoyloxy)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylatehydrochloridehydrate
39. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-(((2z)-2-(2-amino-4-thiazolyl)-1-oxo-2-penten-1-yl)amino)-8-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester, Hydrochloride, Hydrate (1:1:1), (6r,7r)-
40. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-((2-(2-amino-4-thiazolyl)-1-oxo-2- Pentenyl)amino)-8-oxo-, (2,2-dimethyl-1-oxopropoxy)methyl Ester,(6r- (6-.alpha.,7-.beta.(z)))-, Monohydrochloride, Hydrate
41. Pivaloyloxymethyl (+)-(6r,7r)-7-((z)-2-(2-amino-4-thiazolyl)-2-pentenamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid Carbamate Monohydrochloride Monohydrate
| Molecular Weight | 622.1 g/mol |
|---|---|
| Molecular Formula | C23H32ClN5O9S2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 13 |
| Exact Mass | 621.1329977 g/mol |
| Monoisotopic Mass | 621.1329977 g/mol |
| Topological Polar Surface Area | 248 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1060 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |











