

X


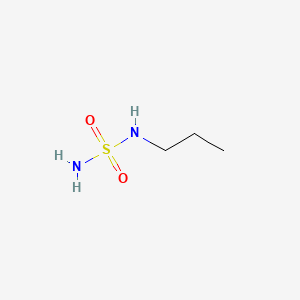

1. 147962-41-2
2. Propylsulfamide
3. 1-(sulfamoylamino)propane
4. Sulfamide, N-propyl-
5. Sulfamide, Propyl-
6. Propyl-sulfamide
7. N-propyl-sulfamide
8. Propyl Sulfuric Diamide
9. N-propylsulfuric Diamide
10. Schembl42318
11. Amy40359
12. Bcp09330
13. Cs-m1494
14. Xfa96241
15. Mfcd12755184
16. Zinc34195342
17. Akos024462413
18. Ds-7778
19. Ac-29731
20. Da-44188
21. Ft-0699977
| Molecular Weight | 138.19 g/mol |
|---|---|
| Molecular Formula | C3H10N2O2S |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 138.04629874 g/mol |
| Monoisotopic Mass | 138.04629874 g/mol |
| Topological Polar Surface Area | 80.6 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 134 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



