

X



1. 183288-42-8
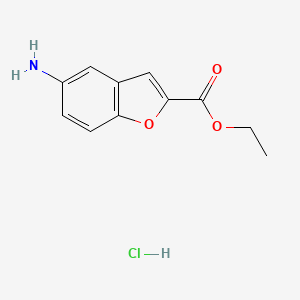
2. Ethyl 5-aminobenzofuran-2-carboxylate Hcl
3. Ethyl 5-amino-1-benzofuran-2-carboxylate Hydrochloride
4. Ethyl 5-amino-1-benzofuran-2-carboxylate;hydrochloride
5. 2-benzofurancarboxylic Acid, 5-amino-, Ethyl Ester, Hydrochloride (1:1)
6. Ethyl 5-amino-1-benzofuran-2-carboxylate Hcl
7. Cs-m2450
8. Cl4582
9. Mfcd22574189
10. Sb21640
11. As-45981
12. Ethyl5-aminobenzofuran-2-carboxylatehydrochloride
13. Ethyl 5-aminobenzofuran-2-carboxylate (hydrochloride)
| Molecular Weight | 241.67 g/mol |
|---|---|
| Molecular Formula | C11H12ClNO3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 241.0505709 g/mol |
| Monoisotopic Mass | 241.0505709 g/mol |
| Topological Polar Surface Area | 65.5 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 244 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |




