

X


1. 2190506-55-7
2. Starbld0008510
3. Schembl19890330
4. Mfcd31735566
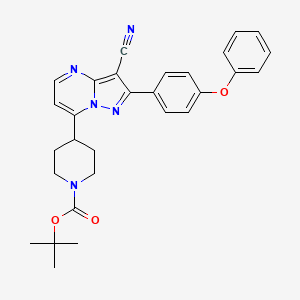
5. 7-(1-boc-4-piperidyl)-2-(4-phenoxyphenyl)pyrazolo[1,5-a]pyrimidine-3-carbonitrile
6. Sy122642
| Molecular Weight | 495.6 g/mol |
|---|---|
| Molecular Formula | C29H29N5O3 |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 92.8 |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 815 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



