

1. (-)-menthol
2. 2216-51-5
3. Levomenthol
4. L-(-)-menthol
5. Menthomenthol
6. Menthacamphor
7. Peppermint Camphor
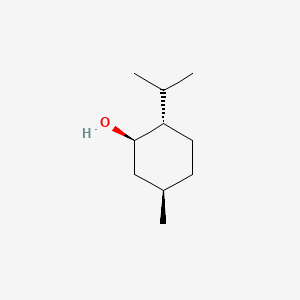
8. (1r,2s,5r)-2-isopropyl-5-methylcyclohexanol
9. U.s.p. Menthol
10. Levomentholum
11. Racementhol
12. (1r,2s,5r)-(-)-menthol
13. (-)-menthyl Alcohol
14. (-)-(1r,3r,4s)-menthol
15. Menthol Racemic
16. Hexahydrothymol
17. (1r)-(-)-menthol
18. D,l-menthol
19. (r)-(-)-menthol
20. 89-78-1
21. D-(-)-menthol
22. Menthol, Dl-
23. I-menthol
24. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1r,2s,5r)-
25. P-menthan-3-ol
26. (-)-trans-p-menthan-cis-ol
27. Rac-menthol
28. 1-menthol
29. (l)-menthol
30. Menthol(-)
31. (1r,2s,5r)-5-methyl-2-(propan-2-yl)cyclohexan-1-ol
32. Menthol, (1r,3r,4s)-(-)-
33. (1r,3r,4s)-(-)-menthol
34. Nci-c50000
35. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1r,2s,5r)-rel-
36. 1r-menthol
37. Nsc 62788
38. Racemic Menthol
39. Water-soluble Menthol
40. (1r,2s,5r)-menthol
41. 5-methyl-2-(1-methylethyl)cyclohexanol
42. (1r,2s,5r)-2-isopropyl-5-methylcyclohexan-1-ol
43. (1r,2s,5r)-rel-2-isopropyl-5-methylcyclohexanol
44. Bz1r15mtk7
45. (1r,2s,5r)-5-methyl-2-(1-methylethyl)cyclohexanol
46. (1r-(1-alpha,2-beta,5-alpha))-5-methyl-2-(1-methylethyl)cyclohexanol
47. (1r,2s,5r)-5-methyl-2-propan-2-ylcyclohexan-1-ol
48. Menthol Crystals
49. Chembl470670
50. Ys08xha860
51. Chebi:15409
52. Menthol Natural
53. Nsc2603
54. (1r,2s,5r)-2-isopropyl-5-methyl-cyclohexanol
55. (1r,2s,5s)-2-isopropyl-5-methyl-cyclohexanol
56. Headache Crystals
57. Nsc-2603
58. Fema No. 2665
59. Nsc-62788
60. Menthol (van)
61. Racementholum
62. Thymomenthol
63. Menthol, Cis-1,3,trans-1,4-
64. Racementol
65. L-menthol (natural)
66. Nsc 2603
67. (+-)-menthol
68. Dsstox_cid_2180
69. Menthol Racemique
70. Levomenthol [inn:ban]
71. Racementhol [inn:ban]
72. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1r-(1.alpha.,2.beta.,5.alpha.)]-
73. Dsstox_rid_75798
74. Dsstox_rid_76516
75. Dsstox_gsid_20805
76. Dsstox_gsid_22180
77. Menthol Natural, Brazilian
78. Menthol, L-
79. Racementol [inn-spanish]
80. Rel-(1r,2s,5r)-2-isopropyl-5-methylcyclohexanol
81. Levomentholum [inn-latin]
82. Menthol Racemique [french]
83. Racementholum [inn-latin]
84. Tra-kill Tracheal Mite Killer
85. (1alpha,2beta,5alpha)-5-methyl-2(1-methylethyl)cyclohexanol
86. Mfcd00062979
87. Cas-89-78-1
88. Ccris 375
89. Cas-2216-51-5
90. L-menthol (tn)
91. Ccris 3728
92. Ccris 4666
93. Hsdb 5662
94. Sr-05000001936
95. (-)-p-menthan-3-ol
96. Einecs 201-939-0
97. Einecs 218-690-9
98. Einecs 239-388-3
99. Unii-bz1r15mtk7
100. Brn 1902288
101. Brn 3194263
102. Levomentol
103. Unii-ys08xha860
104. (+-)-(1r*,3r*,4s*)-menthol
105. Ss-bisabolol
106. Ai3-52408
107. Laevo-menthol
108. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.beta.,5.alpha.)-
109. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1r-(1alpha,2beta,5alpha))-
110. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1r-(1.alpha.,2.beta.,5.alpha.))-
111. L-menthol Natural
112. 1 -menthol
113. Ncgc00159382-02
114. Dextro,laevo-menthol
115. Meggezone
116. Menthol Crystals Usp
117. L-mentholum
118. L-menthol (jp17)
119. Spectrum_000305
120. Levomenthol [ii]
121. Menthol [mi]
122. 5-methyl-2-(1-methylethyl)cyclohexanol, (1alpha,2beta,5alpha)-
123. L-menthol [jan]
124. Menthol, (+/-)-
125. Spectrum2_000855
126. Spectrum3_001561
127. Spectrum5_001060
128. Levomenthol [inn]
129. Racementhol [inn]
130. M0545
131. Dl-menthol [jan]
132. Menthol [who-dd]
133. Menthol,3,trans-1,4-
134. Levomenthol [hsdb]
135. Racementhol [hsdb]
136. Ec 201-939-0
137. Ec 218-690-9
138. Schembl4613
139. Bspbio_003062
140. Kbioss_000785
141. Levomenthol [who-dd]
142. 2-06-00-00052 (beilstein Handbook Reference)
143. 4-06-00-00151 (beilstein Handbook Reference)
144. Mls002207256
145. Divk1c_000820
146. Menthol Racemate [mi]
147. Spectrum1503134
148. Menthol,3r,4s)-(-)-
149. Spbio_000869
150. Gtpl2430
151. Npo-11
152. Dtxsid1020805
153. Dtxsid1022180
154. (-)-menthol, Usp, 97%
155. Hms502i22
156. Kbio1_000820
157. Kbio2_000785
158. Kbio2_003353
159. Kbio2_005921
160. Kbio3_002562
161. Noolisfmxdjskh-kxucptdwsa-
162. (-)-menthol, Analytical Standard
163. Ninds_000820
164. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1r,3r,4s)-
165. Hms1922g13
166. Hms2092l14
167. Hms3885j18
168. Levomenthol [ep Monograph]
169. Pharmakon1600-01503134
170. Cyclohexanol, 2-isopropyl-5-methyl
171. Nsc62788
172. Zinc1482164
173. L-menthol, >=99%, Fcc, Fg
174. Tox21_111620
175. Tox21_201823
176. Tox21_201919
177. Tox21_202608
178. Tox21_302999
179. Tox21_303028
180. Wln: L6tj Ay1&1 Bq D1
181. Bdbm50318482
182. Ccg-40300
183. Cyclohexanol, 2-isopropyl-5-methyl-
184. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1alpha,2beta,5alpha)-
185. Nsc758395
186. S4714
187. Akos016842647
188. (1r, 2s, 5r-)-(-)-menthol
189. Bs-3863
190. Db00825
191. Lmpr0102090001
192. Nsc-758395
193. Sdccgmls-0066659.p001
194. (-)-trans-p-methan-cis-3-ol
195. 1-iso Propyl-4-methyl Cyclohexan-2-ol
196. Idi1_000820
197. Wln: L6tj Ay1&1 Dq D1 -l
198. Ncgc00164247-01
199. Ncgc00164247-02
200. Ncgc00164247-03
201. Ncgc00256525-01
202. Ncgc00256561-01
203. Ncgc00259372-01
204. Ncgc00259468-01
205. Ncgc00260156-01
206. D-(-)-phenylglycine Dane Potassium Salt
207. Fema No. 2665, (-)-
208. Smr001306785
209. L-menthol, Natural, >=99%, Fcc, Fg
210. Sbi-0051777.p002
211. N1950
212. S5868
213. Fema No. 2665, (+/-)-
214. Menthol (racemic) 100 Microg/ml In Methanol
215. (+/-)-(1r*,3r*,4s*)-menthol
216. (1r,2s,5r)-(-)-menthol, Synthetic Pellets
217. C00400
218. Cyclohexanol, (1.alpha.,2.beta.,5.alpha.)-
219. D00064
220. D70313
221. (1r,2r,5s)-2-isopropyl-5-methyl-cyclohexanol
222. Ab00052320_02
223. L-menthol
224. Levomenthol
225. Menthomenthol
226. Menthacamphor
227. (1r,2s,5r)-(-)-menthol, >=99%, Sublimed
228. A843308
229. Q407418
230. Q-201316
231. Sr-05000001936-1
232. Sr-05000001936-2
233. (-)-menthol, Primary Pharmaceutical Reference Standard
234. (1r,2s,5r)-(-)-menthol, Reagentplus(r), 99%
235. 2-isopropyl-5-methylcyclohexanol-, (1r,2s,5r)- #
236. Cyclohexanol, [1r-(1.alpha.,2.beta.,5.alpha.)]-
237. (1r,2s,5r)-5-methyl-2-propan-2-yl-cyclohexan-1-ol
238. Z1698549655
239. (1r,2s,5r)-(-)-menthol, Vetec(tm) Reagent Grade, 98%
240. 6c6a4a8c-a054-468c-a1f0-f29e39838cf2
241. (1r, 2s, 5r)-5-methyl-2-(1-methylethyl)cyclohexyl Alcohol
242. Menthol, United States Pharmacopeia (usp) Reference Standard
243. (1r,2s,5r)-rel-5-methyl-2-(1-methylethyl)cyclohexanol
244. L-menthol, Pharmaceutical Secondary Standard; Certified Reference Material
245. (-)-menthol, Puriss., Meets Analytical Specification Of Ph. Eur., Bp, Usp, 98.0-102.0%
246. (1r-(1-.alpha.,2-.beta.,5-.alpha.))-5-methyl-2-(1-methylethyl)cyclohexanol
247. 114376-98-6
| Molecular Weight | 156.26 g/mol |
|---|---|
| Molecular Formula | C10H20O |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 156.151415257 g/mol |
| Monoisotopic Mass | 156.151415257 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
INHALERS CONTAINING MENTHOL COMPRESSED INTO BLOCKS OR CONES ARE COMMONLY USED FOR THE RELIEF OF NASAL CONGESTION, HEADACHE, AND NEURALGIA. IT IS NOW RARELY ADMINISTERED INTERNALLY. /MENTHOL/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 722
TOPICAL ANTIPRURITIC; MEDICATION (VET): HAS BEEN USED AS A MILD LOCAL ANESTHETIC, ANTISEPTIC & INTERNALLY AS A CARMINATIVE & GASTRIC SEDATIVE /MENTHOL/
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 757
IN LIQUEURS, CONFECTIONERY, PERFUMERY, CIGARETTES, COUGH DROPS, AND NASAL INHALERS /MENTHOL/
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 757
"COOLING" EFFECT OF L-MENTHOL WAS FOUND...TO BE SUPERIOR TO THAT PRODUCED BY OTHER ISOMERS; ODOR AND TASTE, TOO OF L-MENTHOL WERE SUPERIOR, WITH SOME OF THE ISOMERS PRODUCING SHARP, IRRITATING AND DISAGREEABLE PERCEPTIONS.
Osol, A., and R. Pratt. (eds.). The United States Dispensatory. 27th ed. Philadelphia: J.B. Lippincott, 1973., p. 697
Used to treat occasional minor irritation, pain, sore mouth, and sore throat as well as cough associated with a cold or inhaled irritants.
Menthol is a covalent organic compound made synthetically or obtained from peppermint or other mint oils. Menthol induces a cooling sensation on the skin upon inhalation, oral ingestion, or topical application by stimulating the cold-sensitive receptors expressed on the skin, without actually causing a drop in the skin temperature.
...THE PERCENTAGE OF A DOSE OF L-MENTHOL THAT IS EXCRETED COMBINED WITH GLUCURONIC ACID IN THE RABBIT DEPENDS ON THE MAGNITUDE OF THE DOSE; THE LARGER THE DOSE, THE LESS IS THE CONJUGATION.
Opdyke, D.L.J. (ed.). Monographs on Fragrance Raw Materials. New York: Pergamon Press, 1979., p. 519
L-MENTHOL CONJUGATES READILY IN RABBIT FORMING L-MENTHYL-BETA-D-GLUCURONIDE. ABOUT HALF OF THE L-MENTHOL FED TO RABBITS IS EXCRETED COMBINED WITH GLUCURONIC ACID; THE FATE OF OTHER HALF IS NOT KNOWN, BUT IT IS POSSIBLE THAT RING FISSION OCCURS WITH CONSIDERABLE DEGRADATION OF THE MENTHOL MOLECULE.
Opdyke, D.L.J. (ed.). Monographs on Fragrance Raw Materials. New York: Pergamon Press, 1979., p. 519
IN DOGS, MUCH OXIDATION OF MENTHOL TAKES PLACE AND ONLY ABOUT 5% OF THE DOSE CAN BE RECOVERED IN URINE AS THE GLUCURONIDE. /MENTHOL/
Opdyke, D.L.J. (ed.). Monographs on Fragrance Raw Materials. New York: Pergamon Press, 1979., p. 519
L-MENTHOL WAS RAPIDLY BUT INCOMPLETELY GLUCURONIDATED. THE OUTPUT OF L-MENTHOL GLUCURONIDE WAS INCR IN ALL BUT 1 SUBJECT PRETREATED WITH CIMETIDINE (1 G/DAY FOR 1 WK), AN INHIBITOR OF OXIDATIVE DRUG METABOLISM, & IN ALL SUBJECTS PRETREATED WITH A DRUG-METABOLIZING ENZYME INDUCER, PHENOBARBITONE (60 MG NIGHTLY FOR 10 DAYS).
BELL GD ET AL; BR J CLIN PHARMACOL 12 (2): 274 (1981)
Corynebacterium sp. strain RWM1 grew with (-)-menthol, (-)-menthone and other acyclic monoterpenes as sole carbon sources. Growth on menthol was very slow, with a doubling time of more than 24 h, and was not rapid with (-)-menthone (doubling time 12 h). Concentrations of either carbon source greater than 0.025% inhibited growth. (-)-Menthone-grown cultures transiently accumulated 3,7-dimethyl-6-hydroxyoctanoate during growth, and (-)-menthol-grown cells oxidized (-)-menthol, (-)-menthone, 3,7-dimethyl-6-octanolide and 3,7-dimethyl-6-hydroxyoctanoate. Although neither a menthol oxidase nor a menthol dehydrogenase could be detected in extracts of (-)-menthol- or (-)-menthone-grown cells, an induced NADPH-linked monooxygenase with activity towards (-)-menthone was readily detected. With crude cell extracts, only 3,7-dimethyl-6-hydroxyoctanoate was detected as the reaction product. When the (-)-menthone monooxygenase was separated from an induced 3,7-dimethyl-6-octanolide hydrolase by chromatography on hydroxyapatite, the lactone 3,7-dimethyl-6-octanolide was shown to be the product of oxygenation.
Williams DR, PW Trudgill; Microbiology (Reading) 140 (3): 611-6 (1994)
(-)-Menthol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-[(1R,2S,5R)-5-methyl-2-propan-2-ylcyclohexyl]oxyoxane-2-carboxylic acid and p-Menthane-3,-8-diol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Menthol primarily activates the cold-sensitive TRPM8 receptors in the skin. Menthol, after topical application, causes a feeling of coolness due to stimulation of 'cold' receptors by inhibiting Ca++ currents of neuronal membranes. It may also yield analgesic properties via kappa-opioid receptor agonism.
BUILDING BLOCK

MARKET PLACE

