

X

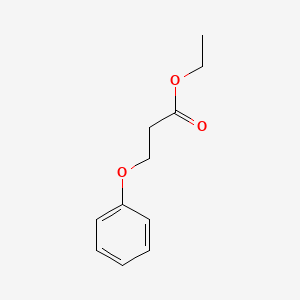

1. Ethyl 3-phenoxypropanoate
2. 22409-91-2
3. Nsc406928
4. Propanoic Acid, 3-phenoxy-ethyl Ester
5. Ethyl3-phenoxypropanoate
6. Schembl38664
7. Dtxsid50324526
8. Txkjopxgysfunc-uhfffaoysa-n
9. Amy25615
10. Mfcd00026919
11. Akos007930362
12. Nsc-406928
13. Propanoic Acid, 3-phenoxy-, Ethyl Ester
14. As-58619
15. Db-045903
16. A816183
17. J-014693
| Molecular Weight | 194.23 g/mol |
|---|---|
| Molecular Formula | C11H14O3 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 35.5 |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 162 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





