
1. Ceflatonin
2. Cephalotaxine
3. Homoharringtonine
4. Homoharringtonine (3(r))-isomer
5. Omacetaxine
6. Synribo
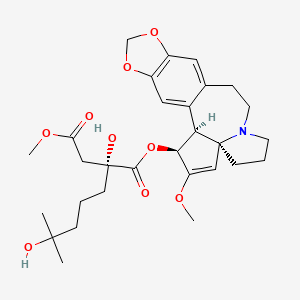
1. Homoharringtonine
2. Ceflatonin
3. Myelostat
4. 26833-87-4
5. Synribo
6. Cgx-635
7. Omapro
8. Homoharringtonin
9. (-)-homoharringtonine
10. Nsc-141633
11. C29h39no9
12. Chebi:71019
13. (2'r,3s,4s,5r)-(-)-homoharringtonine
14. Omacetaxine (homoharringtonine)
15. Omacetaxine Mepesuccinate [usan]
16. 6fg8041s5b
17. Nsc 141633
18. 1-o-[(2s,3s,6r)-4-methoxy-16,18-dioxa-10-azapentacyclo[11.7.0.02,6.06,10.015,19]icosa-1(20),4,13,15(19)-tetraen-3-yl] 4-o-methyl (2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate
19. Cephalotaxine 4-methyl (2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate
20. Omacetaxine Mepesuccinate (usan)
21. Tekinex
22. Synribo (tn)
23. Cephalotaxine, 4-methyl (2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate (ester)
24. Unii-6fg8041s5b
25. Nsc141633
26. Ncgc00025155-01
27. Omacetaxine Mepesuccinate [usan:inn]
28. Brn 5687925
29. Omacetaxini Mepesuccinas
30. Zj-c
31. Mepesuccinate D'omacetaxine
32. Mepesuccinato De Omacetaxina
33. Cephalotaxine 4-methyl (2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate (ester)
34. Mls001424293
35. Chembl46286
36. Homoharringtonine [mi]
37. Cgx-635-14 (formulation)
38. Gtpl7454
39. Schembl12745687
40. Hms3267h22
41. Hms3414n05
42. Hms3678n03
43. Amy33459
44. Homoharringtonine, >=98% (hplc)
45. Bdbm50480293
46. Mfcd05618221
47. Nsc758253
48. S9015
49. Zinc26011099
50. Akos024456585
51. Ccg-269981
52. Db04865
53. Nc00395
54. Nsc-758253
55. Omacetaxine Mepesuccinate [inn]
56. Ncgc00025155-02
57. Ncgc00025155-03
58. Ncgc00025155-04
59. Ncgc00025155-07
60. Omacetaxine Mepesuccinate [mart.]
61. Omacetaxine Mepesuccinate [vandf]
62. Hy-14944
63. Nci60_000917
64. Omacetaxine Mepesuccinate [who-dd]
65. Smr000469230
66. H1775
67. N1504
68. D08956
69. Omacetaxine Mepesuccinate [orange Book]
70. Ab00642561-02
71. Sr-01000597562
72. Q7089373
73. Sr-01000597562-1
74. Brd-k76674262-001-01-7
75. Brd-k76674262-001-02-5
76. Cephalotaxine, 3-[4-methyl (2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate]
77. Cephalotaxine, 4-methyl-, 2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate (ester)
78. (s)-1-((11bs,12s,14ar)-13-methoxy-2,3,5,6,11b,12-hexahydro-1h-[1,3]dioxolo[4',5':4,5]benzo[1,2-d]cyclopenta[b]pyrrolo[1,2-a]azepin-12-yl) 4-methyl 2-hydroxy-2-(4-hydroxy-4-methylpentyl)succinate
79. 1-((1s,3ar,14bs)-2-methoxy-1,5,6,8,9,14b-hexahydro-4h- Cyclopenta(a)(1,3)dioxolo(4,5-h)pyrrolo(2,1-b)(3)benzazepin-1-yl) 4-methyl (2r)-2- Hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate
80. 1-((1s,3ar,14bs)-2-methoxy-1,5,6,8,9,14b-hexahydro-4h-cyclopenta(a)(1,3)dioxolo(4,5-h)pyrrolo(2,1-b)(3)benzazepin-1-yl) 4-methyl (2r)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate
81. Homoharringtonine; Cephalotaxine; [3(r)]-4-methyl 2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate
| Molecular Weight | 545.6 g/mol |
|---|---|
| Molecular Formula | C29H39NO9 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 11 |
| Exact Mass | 545.26248182 g/mol |
| Monoisotopic Mass | 545.26248182 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 968 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in patients who are intolerant and/or resistant to two or more tyrosine kinase inhibitors used to treat accelerated or chronic phase CML.
FDA Label
Philadelphia chromosome positive chronic myeloid leukaemia in patients who have the T315I Bcr-Abl kinase domain mutation and who are resistant to prior imatinib therapy.
The pharmacodynamics of homoharringtonine is not fully understood. It is known that homoharringtonine is involved with protein synthesis inhibition and this leads to its antineoplastic activity.
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX40 - Omacetaxine mepesuccinate
Absorption
Homoharringtonine absorption was not quantified, but maximum concentration is reached after about 30 mins.
Route of Elimination
The main route of elimination for homoharringtonine is still unknown, but renal elimination is less than 15%.
Volume of Distribution
Homoharringtonine has a steady state Vd of 141 93.4 L.
Clearance
Clearance for homoharringtonine was not quantified.
Homoharringtonine has undergoes little hepatic metabolism and is mostly metabolized to 4-DMHHT by plasma esterase hydrolysis.
Homoharringtonine has a half life of about 6 hours after subcutaneous administration.
Homoharringtonine inhibits protein synthesis by not directly binding to Bcr-Abl. It binds to the A-site cleft in the large ribosomal subunit, which affects chain elongation and prevents protein synthesis.
BUILDING BLOCK

