




1. 32454-35-6
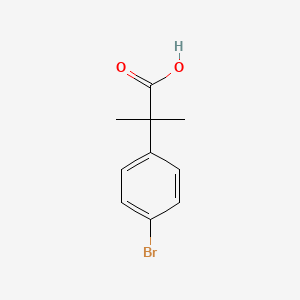
2. 2-(4-bromophenyl)-2-methylpropionic Acid
3. 2-(4-bromophenyl)-2,2'-dimethylacetic Acid
4. Mfcd02684220
5. 2-methyl-2-(p-bromophenyl)propionic Acid
6. Ec 696-555-7
7. Schembl897098
8. Dtxsid70448899
9. Dimethyl(p-bromophenyl)acetic Acid
10. Bcp14118
11. Cs-m1477
12. Zinc2583922
13. Akos009358276
14. Ac-27641
15. Am803697
16. Sy021456
17. Ts-03377
18. 2-(4-bromophenyl)-2-methyl Propionic Acid
19. Db-005775
20. 2-(4-bromo-phenyl)-2-methyl-propionic Acid
21. 2-(4-bromophenyl)-2,2-dimethylacetic Acid
22. Ft-0608593
23. En300-41063
24. 454b356
25. A821280
26. J-509916
27. 2-(4-bromophenyl)-2-methylpropionic Acid, Aldrichcpr
| Molecular Weight | 243.10 g/mol |
|---|---|
| Molecular Formula | C10H11BrO2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 241.99424 g/mol |
| Monoisotopic Mass | 241.99424 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 193 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




