




1. 461432-24-6
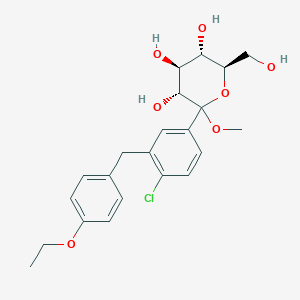
2. Methyl 1-c-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-d-glucopyranoside
3. Schembl1551581
4. Dtxsid601148940
5. Cs-13058
6. Cs-0009474
7. D-glucopyranoside,methyl 1-c-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-
8. Methyl 1-c-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]d-glucopyranoside
9. (3r,4s,5s,6r)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-2-methoxy-tetrahydro-2h-pyran-3,4,5-triol
10. (3r,4s,5s,6r)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)-2-methoxyoxane-3,4,5-triol
| Molecular Weight | 438.9 g/mol |
|---|---|
| Molecular Formula | C22H27ClO7 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 438.1445309 g/mol |
| Monoisotopic Mass | 438.1445309 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 530 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




