
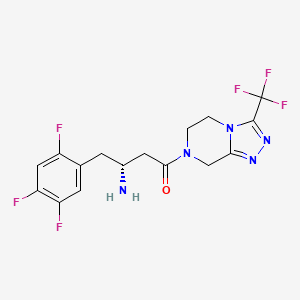

1. 0431, Mk
2. 4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro(1,2,4)triazolo(4,3-a)pyrazin-7(8h)-yl)-1-(2,4,5-trifluorophenyl)butan-2-amine
3. Anhydrous, Sitagliptin Phosphate
4. Januvia
5. Mk 0431
6. Mk-0431
7. Mk0431
8. Monohydrate, Sitagliptin Monophosphate
9. Monohydrate, Sitagliptin Phosphate
10. Monophosphate Monohydrate, Sitagliptin
11. Phosphate Anhydrous, Sitagliptin
12. Phosphate Monohydrate, Sitagliptin
13. Phosphate, Sitagliptin
14. Sitagliptin Monophosphate Monohydrate
15. Sitagliptin Phosphate
16. Sitagliptin Phosphate Anhydrous
17. Sitagliptin Phosphate Monohydrate
1. 486460-32-6
2. Xelevia
3. Mk-0431
4. (r)-3-amino-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one
5. Lez763
6. Qfp0p1dv7z
7. (3r)-3-amino-1-[3-(trifluoromethyl)-5h,6h,7h,8h-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one
8. (3r)-3-amino-1-[3-(trifluoromethyl)-6,8-dihydro-5h-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one
9. (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine
10. Chebi:40237
11. Lez-763
12. 790712-60-6
13. Sitagliptin [inn]
14. Mk0431
15. Sitagliptin (prop.inn)
16. Unii-qfp0p1dv7z
17. Sitagliptina
18. Sitagliptine
19. Sitagliptinum
20. Sr-05000001748
21. Hsdb 7516
22. Lez 763
23. (2r)-4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro(1,2,4)triazolo(4,3-a)pyrazin-7(8h)-yl)-1-(2,4,5-trifluorophenyl)butan-2-amine
24. (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-a Mine
25. Sitagliptin (13)
26. (3r)-3-amino-1-[3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one
27. Sitagliptin; Mk0431
28. Sitagliptin [mi]
29. Sitagliptin [hsdb]
30. Sitagliptin [usan]
31. Ec 690-730-1
32. Sitagliptin [vandf]
33. Chembl1422
34. Schembl17783
35. Bspbio_002262
36. Sitagliptin [who-dd]
37. Triazolopiperazine Analogue 1
38. (3r)-3-amino-1-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-4-(2,4,5-trifluorophenyl)butan-1-one
39. Mls006011959
40. Sitagliptin [ema Epar]
41. Sitagliptin [usan:inn:ban]
42. Gtpl6286
43. Bdbm11162
44. Amy6930
45. Dtxsid70197572
46. 1x70
47. Hms2093f20
48. Act02665
49. Ex-a2816
50. Sitagliptin (metformin,mk-0431)
51. Who 8692
52. Zinc1489478
53. Mfcd09838015
54. Nsc813215
55. Akos015888724
56. Ccg-268731
57. Db01261
58. Nsc-813215
59. Ncgc00178734-03
60. Ncgc00178734-06
61. Ncgc00178734-13
62. 7-((3r)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)buyl)-5,6,7,8-tetrahydro-3-trifluoromethyl)-1,4-triazolo(4,3-a)
63. As-19118
64. Hy-13749
65. Smr002546724
66. Sbi-0206871.p001
67. Sitagliptin 100 Microg/ml In Acetonitrile
68. S5079
69. A14377
70. A25516
71. D08516
72. Ab01563393_01
73. Ar-270/43507782
74. Q419832
75. Q-101366
76. Q-201711
77. Sr-05000001748-1
78. Brd-k19416115-001-01-2
79. Brd-k19416115-001-03-8
80. Z1541638523
81. (1r)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorobenzyl)propylamine
82. (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a] Pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine
83. (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-a
84. (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-alpha]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine
85. (3r)-3-amino-1-[3-(trifluoromethyl)-5h,6h,7h,8h-[1,2,4]triazolo[3,4-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one Hydrochloride
86. (r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine
87. 1,2,4-triazolo(4,3-a)pyrazine, 7-((3r)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl)-5,6,7,8-tetrahydor-3-(trifluoromethyl)-
88. 1,2,4-triazolo(4,3-a)pyrazine-7(8h)-propanamine, 5,6-dihydro-.gamma.-oxo-3-(trifluoromethyl)-.alpha.-((2,4,5-trifluorophenyl)methyl)-, (.alpha.r)-
89. 1,2,4-triazolo[4,3-a]pyrazine,7-[(3r)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-
90. 3-oxo-1-(2,4,5-trifluorobenzyl)-3-(3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl)propylamine
91. 7-[(3r)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine
| Molecular Weight | 407.31 g/mol |
|---|---|
| Molecular Formula | C16H15F6N5O |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 4 |
| Exact Mass | 407.11807909 g/mol |
| Monoisotopic Mass | 407.11807909 g/mol |
| Topological Polar Surface Area | 77 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 566 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | JANUVIA |
| Active Ingredient | SITAGLIPTIN PHOSPHATE |
| Company | MERCK SHARP DOHME (Application Number: N021995. Patents: 6699871, 6890898, 7078381, 7125873, 7326708, 7459428) |
| 2 of 4 | |
|---|---|
| Drug Name | STEGLUJAN |
| Active Ingredient | ERTUGLIFLOZIN; SITAGLIPTIN PHOSPHATE |
| Company | MERCK SHARP DOHME (Application Number: N209805. Patents: 6699871, 6890898, 7078381, 7326708, 7459428, 8080580, 9308204, 9439901) |
| 3 of 4 | |
|---|---|
| Drug Name | JANUMET XR |
| Active Ingredient | METFORMIN HYDROCHLORIDE; SITAGLIPTIN PHOSPHATE |
| Company | MERCK SHARP DOHME (Application Number: N202270. Patents: 6699871, 6890898, 7078381, 7125873, 7326708, 7459428) |
| 4 of 4 | |
|---|---|
| Drug Name | JANUMET |
| Active Ingredient | METFORMIN HYDROCHLORIDE; SITAGLIPTIN PHOSPHATE |
| Company | MERCK SHARP DOHME (Application Number: N022044. Patents: 6699871, 6890898, 7078381, 7125873, 7326708, 7459428, 8414921) |
Hypoglycemic Agents
National Library of Medicine's Medical Subject Headings. Sitagliptin. Online file (MeSH, 2014). Available from, as of July 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Januvia is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. /Included in US product label/
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Januvia should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Type 2 diabetes mellitus is a common chronic disease that causes significant morbidity and mortality worldwide. The primary goal of treatment is to target glycemic control by maintaining the glycosylated hemoglobin level near 6-7% without predisposing patients to hypoglycemia. Diabetes results from a combination of increased hepatic glucose production, decreased insulin secretion from beta cells, and insulin resistance in the peripheral tissues. Currently available antidiabetic agents work by different mechanisms to lower blood glucose levels. Unfortunately, each of them has its tolerability and safety concerns that limit its use and dose titration. Sitagliptin is the first antidiabetic agent from the class of dipeptidyl peptidase-4 enzyme inhibitors. It increases the amount of circulating incretins, which stimulate insulin secretion and inhibit glucose production. Sitagliptin was approved by the US Food and Drug Administration (FDA) for use with diet and exercise to improve glycemic control in adult patients with type 2 diabetes. It can be used alone or in combination with metformin or a thiazolidinedione (pioglitazone or rosiglitazone) when treatment with either drug alone provides inadequate glucose control. The usual adult dose is 100 mg once daily. A dose of 25-50 mg once daily is recommended for patients with moderate-to-severe renal impairment. In randomized, placebo-controlled trials that lasted for up to 6 months, sitagliptin lowered glycosylated hemoglobin levels by 0.5-0.8%. In a 52-week clinical trial, sitagliptin was shown to be noninferior to glipizide as an add-on agent in patients inadequately controlled on metformin alone. Sitagliptin was well tolerated with the most common side effects being gastrointestinal complaints (up to 16%), including abdominal pain, nausea and diarrhea; hypoglycemia and body weight gain occurred at similar rates compared with placebo. Overall, sitagliptin provides a treatment option for patients with type 2 diabetes as a monotherapy, or as an adjunct to metformin or a thiazolidinedione when patients achieve inadequate glycemic control while on either of the agents. It is also an alternative therapy for those patients who have contraindications or intolerability to other antidiabetic agents.
PMID:17700385 Choy M, Lam S; Cardiol Rev 15 (5): 264-71 (2007)
For more Therapeutic Uses (Complete) data for SITAGLIPTIN (6 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: LACTIC ACIDOSIS. Lactic acidosis is a rare, but serious, complication that can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic impairment, renal impairment, and acute congestive heart failure. The onset of lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap, and elevated blood lactate. If acidosis is suspected, Janumet should be discontinued and the patient hospitalized immediately. /Sitagliptin and metformin hydrochloride combination product/
NIH; DailyMed. Current Medication Information for Janumet (Sitagliptin and Metformin Hydrochloride) Tablet, Film-coated, Extended Release (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=d19c7ed0-ad5c-426e-b2df-722508f97d67
FDA is evaluating unpublished new findings by a group of academic researchers that suggest an increased risk of pancreatitis and pre-cancerous cellular changes called pancreatic duct metaplasia in patients with type 2 diabetes treated with a class of drugs called incretin mimetics. These findings were based on examination of a small number of pancreatic tissue specimens taken from patients after they died from unspecified causes. FDA has asked the researchers to provide the methodology used to collect and study these specimens and to provide the tissue samples so the Agency can further investigate potential pancreatic toxicity associated with the incretin mimetics. Drugs in the incretin mimetic class include exenatide (Byetta, Bydureon), liraglutide (Victoza), sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), saxagliptin (Onglyza, Kombiglyze XR), alogliptin (Nesina, Kazano, Oseni), and linagliptin (Tradjenta, Jentadueto). These drugs work by mimicking the incretin hormones that the body usually produces naturally to stimulate the release of insulin in response to a meal. They are used along with diet and exercise to lower blood sugar in adults with type 2 diabetes. FDA has not reached any new conclusions about safety risks with incretin mimetic drugs. This early communication is intended only to inform the public and health care professionals that the Agency intends to obtain and evaluate this new information. ... FDA will communicate its final conclusions and recommendations when its review is complete or when the Agency has additional information to report. The Warnings and Precautions section of drug labels and patient Medication Guides for incretin mimetics contain warnings about the risk of acute pancreatitis. FDA has not previously communicated about the potential risk of pre-cancerous findings of the pancreas with incretin mimetics. FDA has not concluded these drugs may cause or contribute to the development of pancreatic cancer. At this time, patients should continue to take their medicine as directed until they talk to their health care professional, and health care professionals should continue to follow the prescribing recommendations in the drug labels. ...
US FDA; Safety Alerts for Human Medicial Products: Incretin Mimetic Drugs for Type 2 Diabetes: Early Communication - Reports of Possible Increased Risk of Pancreatitis and Pre-cancerous Findings of the Pancreas (Posted March 14, 1013). Available from, as of August 1, 2014: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm343805.htm
Acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis, has been reported during postmarketing experience in patients receiving sitagliptin or sitagliptin/metformin. The most common manifestations associated with pancreatitis were abdominal pain, nausea, and vomiting. Hospitalization was required in 66% of 88 reported cases, including 2 cases of hemorrhagic or necrotizing pancreatitis that necessitated prolonged hospitalization and intensive-care unit (ICU) care. Pancreatitis occurred within 30 days of initiation of sitagliptin or sitagliptin/metformin therapy in 21% of cases; discontinuance of the drug led to resolution of pancreatitis in 53% of patients. At least one other risk factor (e.g., obesity, high cholesterol and/or triglyceride concentrations) was noted in 51% of cases.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3211
Renal function should be assessed prior to initiation of sitagliptin and periodically thereafter. Worsening of renal function, including acute renal failure that sometimes required dialysis, has been reported in some patients during postmarketing experience. A subset of these patients had renal insufficiency, some of whom were prescribed inappropriate dosages of sitagliptin. A return to baseline levels of renal insufficiency has been observed with supportive treatment and discontinuance of potentially causative agents. Cautious reinitiation of sitagliptin can be considered if another etiology is deemed likely to have precipitated the acute worsening of renal function. The manufacturer states that sitagliptin has not been found to be nephrotoxic in clinical trials or in preclinical studies at clinically relevant dosages.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3212
For more Drug Warnings (Complete) data for SITAGLIPTIN (17 total), please visit the HSDB record page.
Sitagliptin is indicated for the management of glycemic control in type 2 diabetes mellitus along with diet and exercise.
FDA Label
For adult patients with type-2 diabetes mellitus, Ristaben is indicated to improve glycaemic control:
- as monotherapy:
- in patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance;
- as dual oral therapy in combination with:
- metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control;
- a sulphonylurea when diet and exercise plus maximal tolerated dose of a sulphonylurea alone do not provide adequate glycaemic control and when metformin is inappropriate due to contraindications or intolerance;
- a peroxisome proliferator-activated-receptor-gamma (PPAR) agonist (i. e. a thiazolidinedione) when use of a PPAR agonist is appropriate and when diet and exercise plus the PPAR agonist alone do not provide adequate glycaemic control;
- as triple oral therapy in combination with:
- a sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control;
- a PPAR agonist and metformin when use of a PPAR agonist is appropriate and when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.
Ristaben is also indicated as add-on to insulin (with or without metformin) when diet and exercise plus stable dose of insulin do not provide adequate glycaemic control.
For adult patients with type-2 diabetes mellitus, Xelevia is indicated to improve glycaemic control:
- as monotherapy:
- in patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance;
- as dual oral therapy in combination with:
- metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control;
- a sulphonylurea when diet and exercise plus maximal tolerated dose of a sulphonylurea alone do not provide adequate glycaemic control and when metformin is inappropriate due to contraindications or intolerance;
- a peroxisome proliferator-activated receptor gamma (PPAR) agonist (i. e. a thiazolidinedione) when use of a PPAR agonist is appropriate and when diet and exercise plus the PPAR agonist alone do not provide adequate glycaemic control;
- as triple oral therapy in combination with:
- a sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control;
- a PPAR agonist and metformin when use of a PPAR agonist is appropriate and when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.
Xelevia is also indicated as add-on to insulin (with or without metformin) when diet and exercise plus stable dose of insulin do not provide adequate glycaemic control.
For patients with type-2 diabetes mellitus, Tesavel is indicated to improve glycaemic control:
- as monotherapy:
- in patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance;
- as dual oral therapy in combination with:
- metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control;
- a sulphonylurea when diet and exercise plus maximal tolerated dose of a sulphonylurea alone do not provide adequate glycaemic control and when metformin is inappropriate due to contraindications or intolerance;
- a PPAR agonist (i. e. a thiazolidinedione) when use of a PPAR agonist is appropriate and when diet and exercise plus the PPAR agonist alone do not provide adequate glycaemic control;
- as triple oral therapy in combination with
- a sulphonylurea and metformin when diet and exercise plus dual therapy with these agents do not provide adequate glycaemic control;
- a peroxisome-proliferator-activated-receptor-gamma (PPAR) agonist and metformin when use of a PPAR agonist is appropriate and when diet and exercise plus dual therapy with these agents do not provide adequate glycaemic control.
Tesavel is also indicated as add on to insulin (with or without metformin) when diet and exercise plus stable dosage of insulin do not provide adequate glycaemic control.
For adult patients with type-2 diabetes mellitus, Januvia is indicated to improve glycaemic control:
- as monotherapy:
- in patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance;
- as dual oral therapy in combination with:
- metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control;
- a sulphonylurea when diet and exercise plus maximal tolerated dose of a sulphonylurea alone do not provide adequate glycaemic control and when metformin is inappropriate due to contraindications or intolerance;
- a peroxisome-proliferator-activated-receptor-gamma (PPAR) agonist (i. e. a thiazolidinedione) when use of a PPAR agonist is appropriate and when diet and exercise plus the PPAR agonist alone do not provide adequate glycaemic control;
- a PPAR agonist (i. e. a thiazolidinedione) when use of a PPAR agonist is appropriate and when diet and exercise plus the PPAR agonist alone do not provide adequate glycaemic control;
- as triple oral therapy in combination with:
- a sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control;
- a PPAR agonist and metformin when use of a PPAR agonist is appropriate and when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.
Januvia is also indicated as add-on to insulin (with or without metformin) when diet and exercise plus stable dose of insulin do not provide adequate glycaemic control.
Treatment of type II diabetes mellitus
For adult patients with type 2 diabetes mellitus, Sitagliptin Accord is indicated to improve glycaemic control:
as monotherapy:
- in patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to contraindications or intolerance.
as dual oral therapy in combination with:
- metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control.
- a sulphonylurea when diet and exercise plus maximal tolerated dose of a sulphonylurea alone do not provide adequate glycaemic control and when metformin is inappropriate due to contraindications or intolerance.
- a peroxisome proliferator-activated receptor gamma (PPARy) agonist (i. e. a thiazolidinedione) when use of a PPARy agonist is appropriate and when diet and exercise plus the PPARy agonist alone do not provide adequate glycaemic control.
as triple oral therapy in combination with:
- a sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.
- a PPARy agonist and metformin when use of a PPARy agonist is appropriate and when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.
Sitagliptin Accord is also indicated as add-on to insulin (with or without metformin) when diet and exercise plus stable dose of insulin do not provide adequate glycaemic control.
Sitagliptin inhibits DPP-4 which leads to increased levels of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide(GIP), decreased levels of glucagon, and a stronger insulin response to glucose.
Dipeptidyl-Peptidase IV Inhibitors
Compounds that suppress the degradation of INCRETINS by blocking the action of DIPEPTIDYL-PEPTIDASE IV. This helps to correct the defective INSULIN and GLUCAGON secretion characteristic of TYPE 2 DIABETES MELLITUS by stimulating insulin secretion and suppressing glucagon release. (See all compounds classified as Dipeptidyl-Peptidase IV Inhibitors.)
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
Incretins
Peptides which stimulate INSULIN release from the PANCREATIC BETA CELLS following oral nutrient ingestion, or postprandially. (See all compounds classified as Incretins.)
A10BH01
A10BH01
A10BH01
A10BH01
A10BH01
A10BH01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BH - Dipeptidyl peptidase 4 (dpp-4) inhibitors
A10BH01 - Sitagliptin
Absorption
Sitagliptin is 87% orally bioavailable and taking it with or without food does not affect its pharmacokinetics. Sitagliptin reaches maximum plasma concentration in 2 hours.
Route of Elimination
Approximately 79% of sitagliptin is excreted in the urine as the unchanged parent compound. 87% of the dose is eliminated in the urine and 13% in the feces.
Volume of Distribution
198L.
Clearance
350mL/min.
Sitagliptin is secreted in the milk of lactating rats at a milk to plasma ratio of 4:1. It is not known whether sitagliptin is excreted in human milk.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Placental transfer of sitagliptin administered to pregnant rats was approximately 45% at 2 hours and 80% at 24 hours postdose. Placental transfer of sitagliptin administered to pregnant rabbits was approximately 66% at 2 hours and 30% at 24 hours.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Approximately 79% of sitagliptin is excreted unchanged in the urine with metabolism being a minor pathway of elimination.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Elimination of sitagliptin occurs primarily via renal excretion and involves active tubular secretion. Sitagliptin is a substrate for human organic anion transporter-3 (hOAT-3), which may be involved in the renal elimination of sitagliptin. The clinical relevance of hOAT-3 in sitagliptin transport has not been established. Sitagliptin is also a substrate of p-glycoprotein, which may also be involved in mediating the renal elimination of sitagliptin. However, cyclosporine, a p-glycoprotein inhibitor, did not reduce the renal clearance of sitagliptin.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
For more Absorption, Distribution and Excretion (Complete) data for SITAGLIPTIN (10 total), please visit the HSDB record page.
Sitagliptin is mostly not metabolised, with 79% of the dose excreted in the urine as the unchanged parent compound. Minor metabolic pathways are mediated mainly by cytochrome p450(CYP)3A4 and to a lesser extent by CYP2C8. After 18 hours, 81% of the dose has remained unchanged, while 2% has been N-sulfated to the M1 metabolite, 6% has been oxidatively desaturated and cyclized to the M2 metabolite, <1% glucuronidated at an unknown site to the M3 metabolite, <1% has been carbamoylated and glucuronidated to the M4 metabolite, 6% has been oxidatively saturated and cyclized to the M5 metabolite, and 2% has been hydroxylated at an unknown site to the M6 metabolite. The M2 metabolite is the cis isomer while the M5 metabolite is the trans isomer of the same metabolite.
The metabolism and excretion of (14)C sitagliptin ... were investigated in humans after a single oral dose of 83 mg/193 muCi. Urine, feces, and plasma were collected at regular intervals for up to 7 days. The primary route of excretion of radioactivity was via the kidneys, with a mean value of 87% of the administered dose recovered in urine. Mean fecal excretion was 13% of the administered dose. Parent drug was the major radioactive component in plasma, urine, and feces, with only 16% of the dose excreted as metabolites (13% in urine and 3% in feces), indicating that sitagliptin was eliminated primarily by renal excretion. Approximately 74% of plasma AUC of total radioactivity was accounted for by parent drug. Six metabolites were detected at trace levels, each representing <1 to 7% of the radioactivity in plasma. These metabolites were the N-sulfate and N-carbamoyl glucuronic acid conjugates of parent drug, a mixture of hydroxylated derivatives, an ether glucuronide of a hydroxylated metabolite, and two metabolites formed by oxidative desaturation of the piperazine ring followed by cyclization. These metabolites were detected also in urine, at low levels. Metabolite profiles in feces were similar to those in urine and plasma, except that the glucuronides were not detected in feces. CYP3A4 was the major cytochrome P450 isozyme responsible for the limited oxidative metabolism of sitagliptin, with some minor contribution from CYP2C8.
PMID:17220239 Vincent SH et al; Drug Metab Dispos 35 (4): 533-8 (2007)
Following a (14)C sitagliptin oral dose, approximately 16% of the radioactivity was excreted as metabolites of sitagliptin. Six metabolites were detected at trace levels and are not expected to contribute to the plasma DPP-4 inhibitory activity of sitagliptin. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of sitagliptin was CYP3A4, with contribution from CYP2C8.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Approximately 12.4 hours. Other studies have reported a half life of approximately 11 hours.
Two double-blind, randomized, placebo-controlled, alternating-panel studies evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of sitagliptin (1.5-600 mg) in healthy male volunteers. Sitagliptin was well absorbed (approximately 80% excreted unchanged in the urine) with an apparent terminal half-life ranging from 8 to 14 hours. ...
PMID:16338283 Herman GA et al; Clin Pharmacol Ther 78 (6): 675-88 (2005)
The apparent terminal half life following a 100 mg oral dose of sitagliptin was approximately 12.4 hours ... .
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
Inhibition of DPP-4 by sitagliptin slows DPP-4 mediated inactivation of incretins like GLP-1 and GIP. Incretins are released throughout the day and upregulated in response to meals as part of glucose homeostasis. Reduced inhibition of incretins increase insulin synthesis and decrease glucagon release in a manner dependant on glucose concentrations. These effects lead to an overall increase in blood glucose control which is demonstrated by reduced glycosylated hemoglobin (HbA1c).
Januvia is a member of a class of oral anti-hyperglycemic agents called dipeptidyl peptidase 4 (DPP-4) inhibitors. The improvement in glycemic control observed with this medicinal product may be mediated by enhancing the levels of active incretin hormones. Incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. Treatment with GLP-1 or with DPP-4 inhibitors in animal models of type 2 diabetes has been demonstrated to improve beta cell responsiveness to glucose and stimulate insulin biosynthesis and release. With higher insulin levels, tissue glucose uptake is enhanced. In addition, GLP-1 lowers glucagon secretion from pancreatic alpha cells. Decreased glucagon concentrations, along with higher insulin levels, lead to reduced hepatic glucose production, resulting in a decrease in blood glucose levels. The effects of GLP-1 and GIP are glucose-dependent such that when blood glucose concentrations are low, stimulation of insulin release and suppression of glucagon secretion by GLP-1 are not observed. For both GLP-1 and GIP, stimulation of insulin release is enhanced as glucose rises above normal concentrations. Further, GLP-1 does not impair the normal glucagon response to hypoglycemia. The activity of GLP-1 and GIP is limited by the DPP-4 enzyme, which rapidly hydrolyzes the incretin hormones to produce inactive products. Sitagliptin prevents the hydrolysis of incretin hormones by DPP-4, thereby increasing plasma concentrations of the active forms of GLP-1 and GIP. By enhancing active incretin levels, sitagliptin increases insulin release and decreases glucagon levels in a glucose-dependent manner. In patients with type 2 diabetes with hyperglycemia, these changes in insulin and glucagon levels lead to lower hemoglobin A1c (HbA1c) and lower fasting and postprandial glucose concentrations. The glucose-dependent mechanism of sitagliptin is distinct from the mechanism of sulfonylureas, which increase insulin secretion even when glucose levels are low and can lead to hypoglycemia in patients with type 2 diabetes and in normal subjects. Sitagliptin is a potent and highly selective inhibitor of the enzyme DPP-4 and does not inhibit the closely-related enzymes DPP-8 or DPP-9 at therapeutic concentrations.
European Medicines Agency (EMEA); European Public Assessment Report for Authorized Medicinal Products for Human Use; Product Information Annex I Summary of Product Characteristics; p.8 (March 21, 2007). Available from, as of August 1, 2014: https://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000722/human_med_000865.jsp&mid=WC0b01ac058001d124
Sitagliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes by slowing the inactivation of incretin hormones. Concentrations of the active intact hormones are increased by Januvia, thereby increasing and prolonging the action of these hormones. Incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme, DPP-4. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. By increasing and prolonging active incretin levels, Januvia increases insulin release and decreases glucagon levels in the circulation in a glucose-dependent manner. Sitagliptin demonstrates selectivity for DPP-4 and does not inhibit DPP-8 or DPP-9 activity in vitro at concentrations approximating those from therapeutic doses.
NIH; DailyMed. Current Medication Information for Januvia (Sitagliptin) Tablet, Film-coated (Revised: February 2014). Available from, as of July 18, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f85a48d0-0407-4c50-b0fa-7673a160bf01
BUILDING BLOCK



LOOKING FOR A SUPPLIER?