


1. Akineton
2. Alpha-bicyclo(2.2.1)hept-5-en-2-yl-alpha-phenyl-1-piperidinepropanol
3. Biperiden Hydrochloride
4. Biperiden, 1r-(1 Alpha,2 Alpha(r*),4 Alpha)-isomer
5. Biperiden, 1s-(1 Alpha,2 Alpha(r*),4 Alpha)-isomer
6. Biperidene
7. Hydrochloride, Biperiden
1. 514-65-8
2. Akineton
3. Biperidene
4. Biperidine
5. Biperidenum
6. Beperiden
7. Biperidene [inn-french]
8. Bipariden
9. Biperideno
10. Biperidenum [inn-latin]
11. Biperideno [inn-spanish]
12. Kl 373
13. Alpha-5-norbornen-2-yl-alpha-phenyl-1-piperidinepropanol
14. Akineton-
15. 1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-phenyl-3-(piperidin-1-yl)propan-1-ol
16. Biperiden Hcl
17. 1-bicycloheptenyl-1-phenyl-3-piperidino-propanol-1
18. 3-piperidino-1-phenyl-1-bicycloheptenyl-1-propanol
19. Nsc-759145
20. Alpha-bicyclo[2.2.1]hept-5-en-2-yl-alpha-phenyl-1-piperidinepropanol
21. 0frp6g56ld
22. Chembl1101
23. Chebi:3112
24. 1-piperidinepropanol, Alpha-5-norbornen-2-yl-alpha-phenyl-
25. 3-piperidino-1-phenyl-1-bicyclo(2.2.1)hepten-(5)-yl-propanol-(1)
26. 1-(5-bicyclo[2.2.1]hept-2-enyl)-1-phenyl-3-piperidin-1-ylpropan-1-ol
27. .alpha.-5-norbornen-2-yl-.alpha.-phenyl-1-piperidinepropanol
28. Biperiden [usan:ban:inn:jan]
29. 1-{bicyclo[2.2.1]hept-5-en-2-yl}-1-phenyl-3-(piperidin-1-yl)propan-1-ol
30. 1-piperidinepropanol, .alpha.-bicyclo[2.2.1]hept-5-en-2-yl-.alpha.-phenyl-
31. Akineton (tn)
32. 1-piperidinepropanol, .alpha.-bicyclo[2.2.1]hept-5-en-2-yl-.alpha.-phenyl-, Hydrochloride
33. Ncgc00182965-01
34. Einecs 208-184-6
35. Unii-0frp6g56ld
36. Brn 0290038
37. Biperiden (jan/usp/inn)
38. Sr-05000001649
39. Hsdb 7639
40. Biperiden [usp:inn:ban:jan]
41. 1-piperidinepropanol, .alpha.-bicyclo(2.2.1)hept-5-en-2-yl-.alpha.-phenyl-
42. 1-(bicyclo(2.2.1)hept-5-en-2alpha-yl)-1-phenyl-3-piperidinopropanol
43. 1-piperidinepropanol, Alpha-bicyclo(2.2.1)hept-5-en-2-yl-alpha-phenyl-
44. Alpha-(bicyclo(2.2.1)hept-5-en-2-yl)-alpha-phenyl-1-piperidino Propanol
45. 3-piperidino-1-phenyl-1-bicyclo(2.2.1)hepten-(5)-yl-propanol-(1) [german]
46. Akinophyl (salt/mix)
47. Biperiden [inn]
48. Biperiden [jan]
49. Biperiden [mi]
50. Biperiden [hsdb]
51. 5-norbornene-2-methanol, Alpha-phenyl-alpha-(2-piperidinoethyl)-
52. Prestwick0_000502
53. Prestwick1_000502
54. Prestwick2_000502
55. Biperiden [vandf]
56. Biperiden [mart.]
57. Biperiden [who-dd]
58. Biperiden [who-ip]
59. Nciopen2_009564
60. Schembl34957
61. 5-20-02-00242 (beilstein Handbook Reference)
62. Spbio_002344
63. Gtpl7128
64. Biperiden [usp Impurity]
65. Dtxsid6022680
66. Schembl20229360
67. Hms2093n17
68. Pharmakon1600-01505514
69. Biperidenum [who-ip Latin]
70. Bdbm50240680
71. Hy-13204a
72. Nsc759145
73. Pdsp1_000821
74. Pdsp2_000808
75. Stl582281
76. 1-(5-bicyclo[2.2.1]hept-2-enyl)-1-phenyl-3-(1-piperidyl)propan-1-ol
77. Akos016008819
78. Ccg-213474
79. Cs-1797
80. Db00810
81. Nsc 759145
82. Sdccgsbi-0206797.p002
83. Ncgc00182965-02
84. Ncgc00182965-03
85. Ncgc00182965-04
86. Ncgc00182965-12
87. Sbi-0206797.p001
88. C07941
89. D00779
90. A918824
91. L001222
92. Q414914
93. Sr-05000001649-1
94. Brd-a00546892-001-01-8
95. A-bicyclo[2.2.1]hept-5-en-2yl-a-phenyl-1-piperidinepropanol
96. 1-piperidinepropanol, .alpha.-5-norbornen-2-yl-.alpha.-phenyl-
97. 1-(2-bicyclo[2.2.1]hept-5-enyl)-1-phenyl-3-piperidin-1-ylpropan-1-ol
98. 1-bicyclo[2.2.1]hept-5-en-2-yl-1-phenyl-3-(1-piperidinyl)-1-propanol #
99. 1-bicyclo[2.2.1]hept-5-en-2-yl-1-phenyl-3-piperidin-1-yl-propan-1-ol
100. 5-norbornene-2-methanol, .alpha.-phenyl-.alpha.-(2-piperidinoethyl)-
101. .alpha.-(bicyclo(2.2.1)hept-5-en-2-yl)-.alpha.-phenyl-1-piperidino Propanol
102. Bicyclo[2.2.1]hept-5-ene-2-methanol, .alpha.-phenyl-.alpha.-[2-(1-piperidinyl)ethyl]-
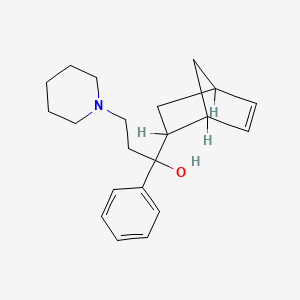
| Molecular Weight | 311.5 g/mol |
|---|---|
| Molecular Formula | C21H29NO |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 311.224914549 g/mol |
| Monoisotopic Mass | 311.224914549 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 422 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Biperiden is used for the adjunctive treatment of all forms of parkinsonian syndrome, in which it appears to be more effective in the postencephalitic and idiopathic than in the arteriosclerotic types. Biperiden usually relieves muscle rigidity, reduces sweating and salivation, and improves gait and, to a lesser extent, tremor. Biperiden also is used for the relief of parkinsonian signs and symptoms of antipsychotic agent-induced (e.g., phenothiazines) extrapyramidal reactions. Although it has been used as adjunctive therapy in the management of other disorders of the extrapyramidal system and of unrelated spastic conditions such as multiple sclerosis, cerebral palsy, and spinal cord injuries, the value of the drug in these conditions requires further investigation.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2661
/EXPL THER/ To study the influence of antidotes on tabun-induced neurotoxicity, the rats were injected intramuscularly with organophosphate tabun. The efficacy of choice antidotal treatment consisting of acetylcholinesterase reactivator obidoxime and one of four anticholinergic drugs (atropine, benactyzine, biperiden, scopolamine) was compared. Testing of tabun-induced neurotoxicity progress was carried out using the method Functional observational battery. The experimental animals as well as controls were observed at 24 hours and 7 days following tabun or saline administration. The results were compared to the condition of animals without anticholinergic drug (oxime alone) and control rats that received physiological solution instead of tabun and treatment. Antidotal treatment involving centrally acting anticholinergic drugs (benactyzine, biperiden, scopolamine) showed significantly higher neuroprotective efficacy compared to antidotal treatment containing atropine.
PMID:15168875 Krejcova G, Kassa J; Acta Medica (Hradec Kralove) 47 (1): 13-8 (2004)
/EXPL THER/ To study the influence of pharmacological pretreatment (PANPAL or pyridostigmine combined with biperiden) and antidotal treatment (the oxime HI-6 plus atropine) on soman-induced neurotoxicity, male albino rats were poisoned with a lethal dose of soman (54 (g/kg im; 100% of LD50 value) and observed at 24 hours and 7 days following soman challenge. The neurotoxicity of soman was evaluated using a Functional observational battery and an automatic measurement of motor activity. Pharmacological pretreatment as well as antidotal treatment were able to eliminate some of soman-induced neurotoxic effects observed at 24 hours following soman poisoning. The combination of pharmacological pretreatment (PANPAL or pyridostigmine combined with biperiden) and antidotal treatment was found to be more effective in the elimination of soman-induced neurotoxicity in rats at 24 hours following soman challenge in comparison with the administration of pharmacological pretreatment or antidotal treatment alone. To compare both pharmacological pretreatments, the combination of pyridostigmine with biperiden seems to be more efficacious to eliminate soman-induced signs of neurotoxicity than PANPAL. At 7 days following soman poisoning, the combination of pharmacological pretreatment involving pyridostigmine and biperiden with antidotal treatment was only able to completely eliminate soman-induced neurotoxic signs. Thus, our findings confirm that the combination of pharmacological pretreatment and antidotal treatment is able not only to protect the experimental animals from the lethal effects of soman, but also to eliminate most soman-induced signs of neurotoxicity in poisoned rats. The pharmacological pretreatment containing pyridostigmine and biperiden appears to be more efficacious to eliminate soman-induced neurotoxic sings than PANPAL.
PMID:14677718 Kassa J et al; Acta Medica (Hradec Kralove) 46 (3): 101-7 (2003)
Isolated instances of mental confusion, euphoria, agitation and disturbed behavior have been reported in susceptible patients. Also, the central anticholinergic syndrome can occur as an adverse reaction to properly prescribed anticholinergic medication, although it is more frequently due to overdosage. It may also result from concomitant administration of an anticholinergic agent and a drug that has secondary anticholinergic actions. Caution should be observed in patients with manifest glaucoma, though no prohibitive rise in intraocular pressure has been noted following either oral or parenteral administration. Patients with prostatism, epilepsy or cardiac arrhythmia should be given this drug with caution. Occasionally, drowsiness may occur, and patients who drive a car or operate any other potentially dangerous machinery should be warned of this possibility. As with other drugs action on the central nervous system, the consumption of alcohol should be avoided during Akineton therapy.
US Natl Inst Health; DailyMed. Current Medication Information for Akineton - Biperiden hydrochloride (June 2006). Available from, as of October 21, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1093
Biperiden should be used with caution or may be contraindicated in patients with conditions in which anticholinergic effects are undesirable. The usual precautions and contraindications associated with antimuscarinics should be observed with biperiden.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2662
Adverse reactions to biperiden are mainly extensions of its anticholinergic effects. Dryness of the mouth and blurred vision are common and are dose related. GI disturbances may occur and can be alleviated by administering the drug with or after meals. Drowsiness, dizziness, and mental confusion occur less frequently. Transient psychotic reactions, euphoria or disorientation, agitation, disturbed behavior, urinary retention, and hematuria have been reported rarely. In some severe cases of parkinsonian syndrome, tremor may increase as spasticity is relieved. In addition, generalized choreiform movements have been reported in at least one patient with parkinsonian syndrome when biperiden was added to levodopa-carbidopa therapy. A reduction in rapid eye movement (REM) sleep, characterized by increased REM latency and decreased percentage of time spent in REM sleep, also has been reported.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2661-2
It is not known whether biperiden is distributed into milk. Because many drugs are distributed into milk, biperiden should be used with caution in nursing women.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2662
For more Drug Warnings (Complete) data for BIPERIDEN (8 total), please visit the HSDB record page.
For use as an adjunct in the therapy of all forms of parkinsonism and control of extrapyramidal disorders secondary to neuroleptic drug therapy.
Biperiden is a weak peripheral anticholinergic agent. It has, therefore, some antisecretory, antispasmodic and mydriatic effects. In addition, biperiden possesses nicotinolytic activity. The parenteral form of biperiden is an effective and reliable agent for the treatment of acute episodes of extrapyramidal disturbances sometimes seen during treatment with neuroleptic agents. Akathisia, akinesia, dyskinetic tremors, rigor, oculogyric crisis, spasmodic torticollis, and profuse sweating are markedly reduced or eliminated. With parenteral biperiden, these drug-induced disturbances are rapidly brought under control.
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
N04AA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N04 - Anti-parkinson drugs
N04A - Anticholinergic agents
N04AA - Tertiary amines
N04AA02 - Biperiden
Absorption
87% bioavailability
The serum concentration at 1 to 1.5 hours following a single, 4 mg oral dose was 4-5 ng/mL. Plasma levels (0.1-0.2 ng/mL) could be determined up to 48 hours after dosing. Six hours after an oral dose of 250 mg/kg in rats, 87% of the drug had been absorbed.
US Natl Inst Health; DailyMed. Current Medication Information for Akineton - Biperiden hydrochloride (June 2006). Available from, as of October 21, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1093
The subcellular distribution of biperiden (BP), trihexyphenidyl (TP) and (-)-quinuclidinyl benzylate (QNB) in brain, heart and lung following high dose (3.2 mg/kg) iv administration was investigated in rats. The subcellular distribution of BP or TP used clinically conformed with that of QNB, a typical potent central muscarinic antagonist. The concentration-time courses of the brain subcellular fractions for these drugs were of two types which decreased slowly and in parallel to the plasma concentration. The subcellular distribution in the brain and heart was dependent on the protein amount of each fraction. The percent post-nuclear fraction (P2) of the total concentration in the lung was characteristically about 3-5 times larger than that in the heart. It was elucidated that the distribution in the lung differs from that in the brain and heart, with high affinity which is not dependent on the protein amount in the P2 fraction containing lysosomes. On the other hand, at a low dose (650 ng/kg) of 3H-QNB, each fraction as a percentage of the total concentration in the brain increased in synaptic membrane and synaptic vesicles and decreased in nuclei and cytosol as compared with the high dose. These results show that although the tissue concentration-time courses of anticholinergic drugs appear to decrease simply in parallel to plasma concentration, the subcellular distribution exhibits a variety of patterns among various tissues.
PMID:9477171 Ishizaki J et al; Biol Pharm Bull 21 (1): 67-71(1998)
The pharmacokinetics of biperiden were studied and compared with pharmacodynamics (pupil size, accommodation, self-rating mood scale) in 6 healthy volunteers. A single-blind cross-over design was employed with placebo and biperiden (4 mg as commercially available tablets). After a lag time of 0.5 hr, biperiden was rapidly absorbed with a half-life of 0.3 hr, plasma peak levels of 5 ng/mL being reached after 1.5 hr. Biperiden showed good tissue penetration (distribution half-life 0.6 hr; ratio of total to central distribution volume 9.6), the terminal half-life time of plasma concentration was 18 hr, and the oral clearance was 146 L/hr. The pharmacodynamic maximum lagged behind the plasma peak concentration by 1 (self-rating) to 4 hr (accommodation).
PMID:6519170 Hollmann M et al; Eur J Clin Pharmacol 27 (5): 619-21 (1984)
The metabolism of biperiden is not completely understood, but does involve hydroxylation.
A single-blind cross-over design was employed with placebo and biperiden (4 mg as commercially available tablets). After a lag time of 0.5 hr, biperiden was rapidly absorbed with a half-life of 0.3 hr, ... Biperiden showed good tissue penetration (distribution half-life 0.6 hr; ratio of total to central distribution volume 9.6), the terminal half-life time of plasma concentration was 18 hr, ... .
PMID:6519170 Hollmann M et al; Eur J Clin Pharmacol 27 (5): 619-21 (1984)
Parkinsonism is thought to result from an imbalance between the excitatory (cholinergic) and inhibitory (dopaminergic) systems in the corpus striatum. The mechanism of action of centrally active anticholinergic drugs such as biperiden is considered to relate to competitive antagonism of acetylcholine at cholinergic receptors in the corpus striatum, which then restores the balance.
Akineton is a weak peripheral anticholinergic agent. It has, therefore, some antisecretory, antispasmodic and mydriatic effects. In addition, Akineton possesses nicotinolytic activity. Parkinsonism is thought to result from an imbalance between the excitatory (cholinergic) and inhibitory (dopaminergic) systems in the corpus striatum. The mechanism of action of centrally active anticholinergic drugs such as Akineton is considered to relate to competitive antagonism of acetylcholine at cholinergic receptors in the corpus striatum, which then restores the balance.
US Natl Inst Health; DailyMed. Current Medication Information for Akineton - Biperiden hydrochloride (June 2006). Available from, as of October 21, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1093
Like other antimuscarinic agents of the trihexyphenidyl group, biperiden has an atropine-like blocking action on parasympathetic-innervated peripheral structures, including smooth muscle. In addition to antispasmodic, antisecretory, and mydriatic effects, biperiden has an antinicotinic potency about 6 times that of atropine and 5 times that of trihexyphenidyl on a weight basis in experimental animals.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2662
BUILDING BLOCK



LOOKING FOR A SUPPLIER?
