


1. Cp 690,550
2. Cp 690550
3. Cp-690,550
4. Cp-690550
5. Cp690550
6. Tasocitinib
7. Tofacitinib
8. Xeljanz
1. 540737-29-9
2. Tasocitinib Citrate
3. Xeljanz
4. Cp-690550 Citrate
5. Tofacitinib (citrate)
6. Tofacitinib (cp-690550) Citrate
7. Xeljanz Xr
8. Tofacitinib Citrate [usan]
9. Cp-690,550-10
10. Tasocitinib Monocitrate
11. 540737-29-9 (citrate)
12. Cp-690550-10
13. Cp 690550 Citrate
14. O1ff4div0d
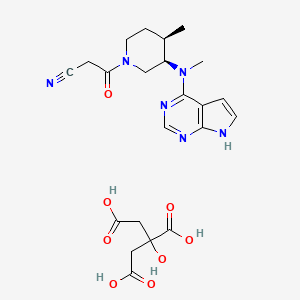
15. 3-((3r,4r)-4-methyl-3-(methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile 2-hydroxypropane-1,2,3-tricarboxylate
16. Chebi:71197
17. 1-piperidinepropanenitrile, 4-methyl-3-(methyl-7h-pyrrolo(2,3-d)pyrimidin-4-ylamino)- Beta-oxo-, (3r,4r)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
18. 2-hydroxypropane-1,2,3-tricarboxylic Acid; 3-[(3r,4r)-4-methyl-3-[methyl({7h-pyrrolo[2,3-d]pyrimidin-4-yl})amino]piperidin-1-yl]-3-oxopropanenitrile
19. 2-hydroxypropane-1,2,3-tricarboxylic Acid;3-[(3r,4r)-4-methyl-3-[methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile
20. Unii-o1ff4div0d
21. Mfcd11616529
22. 3-{(3r,4r)-4-methyl-3-[methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl}-3-oxopropanenitrile 2-hydroxypropane-1,2,3-tricarboxylate
23. 3-{(3r,4r)-4-methyl-3-[methyl-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile Citrate Salt
24. 3-{(3r,4r)-4-methyl-3-[methyl-(7h-pyrrolo[2,3-d]pyrimidin-4-yl}-amino]-piperidin-1-yl)-3-oxo-propionitrile Citrate Salt
25. Xeljanz (tn)
26. Tofacitinib Monocitrate
27. Tasocitinib Citric Acid Salt
28. Cep-18770(delanzomib)
29. Mls006010058
30. Schembl1374185
31. Chembl2103743
32. Tofacitinib Citrate (jan/usan)
33. Amy4175
34. Dtxsid80202404
35. Ex-a204
36. Tofacitinib Citrate [jan]
37. C16h20n6o.c6h8o7
38. Tofacitinib Monocitrate [mi]
39. Hy-40354a
40. S5001
41. Tofacitinib Citrate [who-dd]
42. Akos022178222
43. Cp-690550 - Tofacitinib Citrate
44. Tofacitinib Citrate, >=98% (hplc)
45. Ccg-269730
46. Cs-0928
47. Tofacitinib Citrate [orange Book]
48. Ac-25004
49. As-19392
50. Bt163661
51. Smr004701220
52. Cp-690550 Citrate (tofacitinib Citrate)
53. Tofacitinib Citrate (cp-690550 Citrate)
54. Cp 690550-10
55. D09783
56. Q27139435
57. (3r,4r)-4-methyl-3-(methyl-7h-pyrro Lo[2,3-d]pyrimidin-4-ylamino)-beta-oxo-1-piperidinepro Panenitrile Citrate
58. (3r,4r)-4-methyl-3-(methyl-7h-pyrrolo[2,3-d]pyrimidin-4-ylamino)-?-oxo-1-piperidinepropanenitrile Citrate
59. (3r,4r)-4-methyl-3-(methyl-7h-pyrrolo[2,3-d]pyrimidin-4-ylamino)-beta-oxo-1-piperidine Propanenitrile 2-hydroxy-1,2,3-propanetricarboxylate
60. (3r,4r)-4-methyl-3-(methyl-7h-pyrrolo[2,3-d]pyrimidin-4-ylamino)-ss-oxo-1-piperidinepropanenitrile Citrate Salt
61. 1-piperidinepropanenitrile, 4-methyl-3-(methyl-7h-pyrrolo(2,3-d)pyrimidin-4-ylamino)-.beta.-oxo-, (3r,4r)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
62. 1-piperidinepropanenitrile,4-methyl-3-(methyl-7h-pyrrolo[2,3-d]pyrimidin-4-ylamino)-beta-oxo-,(3r,4r)-,2-hydroxy-1,2,3-propanetricarboxylate(1:1)
63. 3-((3r,4r)-4-methyl-3-(methyl(7h-pyrrolo(2,3-d)pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxo-propanenitrile2-hydroxypropane-1,2,3-tricarboxylate (1:1)
64. 3-((3r,4r)-4-methyl-3-(methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile Mono Citrate Salt
65. 3-{(3r,4r)-4-methyl-3-[methyl-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile Mono Citrate Salt
| Molecular Weight | 504.5 g/mol |
|---|---|
| Molecular Formula | C22H28N6O8 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Exact Mass | 504.19686187 g/mol |
| Monoisotopic Mass | 504.19686187 g/mol |
| Topological Polar Surface Area | 221 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 716 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | XELJANZ XR |
| Active Ingredient | TOFACITINIB CITRATE |
| Company | PFIZER INC (Application Number: N208246. Patents: 6956041, 6965027, 7091208, 7265221, 7301023, RE41783) |
| 2 of 2 | |
|---|---|
| Drug Name | XELJANZ |
| Active Ingredient | TOFACITINIB CITRATE |
| Company | PF PRISM CV (Application Number: N203214. Patents: 6956041, 6965027, 7091208, 7265221, 7301023, RE41783) |
Janus Kinase Inhibitors
Agents that inhibit JANUS KINASES. (See all compounds classified as Janus Kinase Inhibitors.)
BUILDING BLOCK



LOOKING FOR A SUPPLIER?
