


1. B10 9359
2. B10-9359
3. B109359
4. Ethyl Etrinoate
5. Etrinoate, Ethyl
6. Ro 10 9359
7. Ro 10-9359
8. Ro 109359
9. Ro-10-9359
10. Ro109359
11. Tigason
12. Tigazon
1. 54350-48-0
2. Tegison
3. Ethyl Etrinoate
4. Retinoid
5. Etretinato
6. Ro 10-9359
7. Etretinatum
8. Tigason
9. Ro-109359
10. Chebi:4913
11. Ro-10-9359
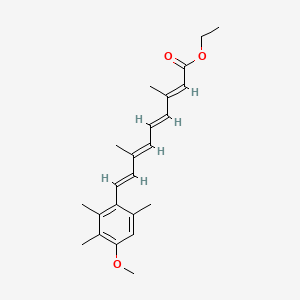
12. Ethyl (2e,4e,6e,8e)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate
13. Nsc-297936
14. 65m2udr9ag
15. Acitretin Related Compound B
16. Ethyl (all-e)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
17. Ethyl All-trans-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
18. 3,7-dimethyl-9-(4-methoxy-2,3,6-trimethylphenyl)-2,4,6,8-nonanetetraenoic Acid Ethyl Ester
19. Ncgc00167500-01
20. Dsstox_cid_3036
21. 2,4,6,8-nonatetraenoic Acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-, Ethyl Ester, (all-e-)
22. Dsstox_rid_76843
23. Dsstox_gsid_23036
24. Etretinatum [inn-latin]
25. Etretinato [inn-spanish]
26. (2e,4e,6e,8e)-9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-tetraenoic Acid Ethyl Ester
27. Cas-54350-48-0
28. Ccris 3615
29. Hsdb 7185
30. Sr-05000001511
31. Einecs 259-119-3
32. Unii-65m2udr9ag
33. Nsc 297936
34. Etretinate [usan:inn:ban:jan]
35. 2,4,6,8-nonatetraenoic Acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, Ethyl Ester, (all-e)-
36. Etretinate [mi]
37. Etretinate [inn]
38. Etretinate [jan]
39. Tegison (tn)
40. Etretinate [hsdb]
41. Etretinate [usan]
42. Etretinate [vandf]
43. Chembl464
44. Etretinate [mart.]
45. Schembl3123
46. Etretinate [who-dd]
47. Etretinate (jan/usan/inn)
48. Gtpl7599
49. Dtxsid0023036
50. Etretinate [orange Book]
51. Chebi:94591
52. 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic Acid Ethyl Ester
53. Hms2090g06
54. Hms3713f22
55. Hy-b0797
56. Zinc3830820
57. Tox21_112501
58. Bdbm50248000
59. Lmpr01090046
60. Mfcd00866624
61. Nsc297936
62. S4699
63. Akos015889992
64. Tox21_112501_1
65. Ac-6823
66. Ccg-220590
67. Cs-3926
68. Ncgc00167500-02
69. Ro-13-7837
70. 2,4,6,8-nonanetetraenoic Acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, Ethyl Ester, All-trans-
71. 2,4,6,8-nonatetraenoic Acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, Ethyl Ester, (2e,4e,6e,8e)-
72. As-77381
73. Etretinate 100 Microg/ml In Acetonitrile
74. Acitretin Impurity B [ep Impurity]
75. E1293
76. Acitretin Related Compound B [usp-rs]
77. A16356
78. D00316
79. Ab01275503-01
80. 350e480
81. A830117
82. Isopropyl-pyridin-4-yl-aminedihydrochloride
83. Q554297
84. Acitretin Related Compound B [usp Impurity]
85. Sr-05000001511-1
86. Sr-05000001511-2
87. W-105640
88. Brd-k36248164-001-01-8
89. Ethyl 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
90. Ethyl 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate
91. (2e,4e,6e,8e)-ethyl 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate
92. 2,6,8-nonatetraenoic Acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, Ethyl Ester, (all E)-
93. 2,6,8-nonatetraenoic Acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, Ethyl Ester, (all-e)-
94. Ethyl (2e,4e,6e,8e)-3,7-dimethyl-9-[2,3,6-trimethyl-4-(methyloxy)phenyl]nona-2,4,6,8-tetraenoate
95. Ethyl (2e,4e,6e,8e)-9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-tetraenoate;etretinate
| Molecular Weight | 354.5 g/mol |
|---|---|
| Molecular Formula | C23H30O3 |
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 354.21949481 g/mol |
| Monoisotopic Mass | 354.21949481 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 568 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsoriatic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 689
Etretinate is indicated for the treatment of severe recalcitrant psoriasis, including the erythrodermic and generalized pustular types, in patients who are unresponsive to or intolerant of the standard therapies. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1330
Etretinate is used for the treatment of severe, intractable oral lichen planus. /NOT included in use product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1330
Etretinate is also used in correcting severe intractable forms of keratinization disorders, such as: dermatoses, ichthyosiform; erythroderma, congenital ichthyosiform; ichthyosis, lamellar, and other ichthyoses; keratosis follicularis (Darier's disease); keratosis palmaris et plantaris; pityriasis rubra pilaris (PRP); pustulosis, palmoplanter. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1330
Pregnancy risk category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweights any possible benefit to the patient./
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
Etretinate is contraindicated during pregnancy, since it has caused major human fetal abnormalities, including meningomyelocele; meningoencephalocoele; multiple synostoses; facial dysmorphia; syndactyly; absence of terminal phalanges; malformations of hip, ankle, and forearm; abnormalities of the heart and thymus; low set ears; high palate; decreased cranial volume; and alterations of the skull and cervical vertebrae.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
... It has not been determined how long pregnancy should be avoided after discontinuation of treatment; patients have been followed for a long as 2 years after treatment was discontinued, and fetal abnormalities associated with etretinate have occurred during this 2 year period. Therefore, etretinate should not be used in women who plan to have children in the future.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
In women of childbearing potential, etretinate should not be used until the possibility of pregnancy is ruled out. In addition, etretinate should not be used in women who, while undergoing treatment and for an indefinite period of time thereafter, are deemed unreliable in their use of contraception or who may not use reliable contraception.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
For more Drug Warnings (Complete) data for ETRETINATE (13 total), please visit the HSDB record page.
Keratolytic Agents
Agents that soften, separate, and cause desquamation of the cornified epithelium or horny layer of skin. They are used to expose mycelia of infecting fungi or to treat corns, warts, and certain other skin diseases. (See all compounds classified as Keratolytic Agents.)
D - Dermatologicals
D05 - Antipsoriatics
D05B - Antipsoriatics for systemic use
D05BB - Retinoids for treatment of psoriasis
D05BB01 - Etretinate
Concentrations of etretinate and its active metabolite in epidermal specimens obtained after 1 to 36 months of therapy were a function of location; subcutis much greater than serum greater than epidermis greater than dermis.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
Etretinate accumulates in high concentrations in adipose tissue, especially in the liver and in subcutaneous fat. Liver concentrations of etretinate in patients who had received therapy for six months were generally higher than accompanying plasma concentrations and tended to be higher still in livers with a higher degree of fatty infiltration.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
Studies in normal volunteers indicate that the absorption of etretinate is greater in patients consuming whole milk or a high-fat diet than in patients in a fasting state.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
Etretinate is absorbed in the small intestine.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
For more Absorption, Distribution and Excretion (Complete) data for ETRETINATE (8 total), please visit the HSDB record page.
The aromatic retinoid acitretin is the primary active metabolite of etretinate, and in this study the ethyl esterification of acitretin to etretinate using [(14)C]acitretin and human liver microsomes /was investigated/. ... This study demonstrated that in the presence of ethanol the ethyl esterification of acitretin to etretinate proceeds via formation of acitretinoyl-CoA. Predicting clearance of acitretin in vivo via this unique metabolic pathway will be a challenge, as the intracellular concentration of ethanol could never be predicted with any degree of accuracy in humans.
PMID:10874125 Knights KM et al; Biochem Pharmacol 60 (4): 507-16 (2000)
In one study, the apparent terminal half life of etretinate after 6 months of therapy was approximately 120 days.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1331
BUILDING BLOCK



LOOKING FOR A SUPPLIER?
