
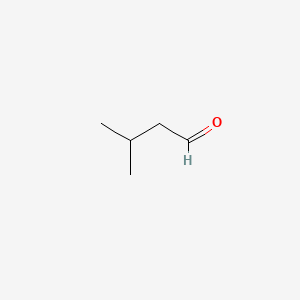

1. 3-methylbutanal
2. Isopentanal
3. Isovalerylaldehyde
1. 3-methylbutanal
2. 590-86-3
3. 3-methylbutyraldehyde
4. Isovaleral
5. Isopentaldehyde
6. Isoamylaldehyde
7. Isopentanal
8. Butanal, 3-methyl-
9. Isovalerylaldehyde
10. Isovaleric Aldehyde
11. Beta-methylbutanal
12. Isoamyl Aldehyde
13. 2-methylbutanal-4
14. 3-methyl-1-butanal
15. 1-butanal, 3-methyl-
16. Butyraldehyde, 3-methyl-
17. Iso-c4h9cho
18. Iso-valeraldehyde
19. 3-methylbutylaldehyde
20. Beta-methylbutyraldehyde
21. 3-methyl Butanal
22. Fema No. 2692
23. Butanal, Methyl-
24. 3-methyl-butanal
25. Nsc 404119
26. 3-methylbutan-1-al
27. 3-methyl Butyraldehyde
28. 3-methyl-butyraldehyde
29. Aldehyde Isovalerianique
30. Ccris 2945
31. Hsdb 628
32. 3-methylbutyraldehyde (natural)
33. Einecs 209-691-5
34. Brn 0773692
35. .beta.-methylbutanal
36. Dtxsid1021619
37. Unii-69931rwi96
38. Chebi:16638
39. Ai3-16106
40. Isovaleric-aldehyde
41. 69931rwi96
42. Nsc-404119
43. Chembl18360
44. Dtxcid201619
45. Ec 209-691-5
46. 4-01-00-03291 (beilstein Handbook Reference)
47. Mfcd00007014
48. 26140-47-6
49. Aldehyde Isovalerianique [french]
50. B-methylbutanal
51. Methyl Butanal
52. Isovaler Aldehyde
53. Isovaleraldehyde, 97%
54. Isopentanal [inci]
55. Isovaleraldehyde [mi]
56. Isovaleraldehyde [hsdb]
57. Wln: Vh1y1&1
58. 3-methyl Butanal [fcc]
59. Isovaleraldehyde, >=97%, Fg
60. Str03918
61. Isovaleraldehyde, Analytical Standard
62. Tox21_200891
63. 3-methylbutyraldehyde [fhfi]
64. Bdbm50028832
65. Nsc404119
66. Akos000118930
67. Isovaleraldehyde, Natural, >=95%, Fg
68. Ncgc00248867-01
69. Ncgc00258445-01
70. Cas-590-86-3
71. Pd124039
72. Ft-0627530
73. I0192
74. Ns00006828
75. En300-18032
76. C07329
77. Q409554
78. J-512894
79. F2190-0631
80. Inchi=1/c5h10o/c1-5(2)3-4-6/h4-5h,3h2,1-2h
| Molecular Weight | 86.13 g/mol |
|---|---|
| Molecular Formula | C5H10O |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 17.1 |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 39.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Aldehydes are readily oxidized to organic acids, which, in turn, can serve as substrates for fatty acid oxidation pathways and the Krebs cycle. ... Oxidation of aldehydes is catalyzed by aldehyde dehydrogenase, which has been found in the brain, erythrocytes, liver, kidney, heart, and placenta. /Aldehydes/
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2635
... The detoxification of aldehydes can be seen to proceed basically via two routes: (1) an oxidation to yield readily metabolized acids; (2) inactivation by reaction with sulfhydryl groups, particularly glutathione. Under conditions that either deplete glutathione levels, or that result in an inhibition of aldehyde dehydrogenase (for example, Antabuse treatment), the acute and chronic effects of aldehyde toxicity might be more fully expressed. /Aldehydes/
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2637
3-Methylbutanal is a volatile aldehyde which in the rat is derived, at least in part, from colonic bacterial breakdown of leucine. It has been proposed as a toxin in the pathogenesis of hepatic encephalopathy in man. The mean plasma 3-methylbutanal concentration in non-fasting patients with hepatic encephalopathy, 0.244 umol/liter (range 0-1.30) was not significantly different from the mean value in controls, 0.116 umol/liter (0-0.349). Oral leucine feeding resulted in significant increases in plasma 3-methylbutanal concentrations in both control subjects and patients with cirrhosis. Peak leucine and 3-methylbutanal values occurred at approximately the same time and usually within 120 min of leucine ingestion. Pre-treatment with neomycin had no effect on the results of leucine feeding. No changes occurred in the clinical condition or psychometric performance of patients with cirrhosis fed leucine despite increases in plasma 3-methylbutanal of up to 700% over basal values. In man plasma 3-methylbutanal, at least in part, derives from ingested leucine independently of the action of colonic bacteria.
PMID:4038637 Marshall AW et al; Clin Physiol 5 (1): 53-62 (1985)
The cytochrome P450-catalyzed formation of olefinic products from a series of xenobiotic aldehydes has been demonstrated. Isobutyraldehyde and trimethylacetaldehyde, but not propionaldehyde, are converted to the predicted olefinic products, suggesting a requirement for branching at the alpha carbon. In addition, the four C5 aldehydes of similar hydrophobicity were compared for their ability to undergo the reaction. The straight-chain valeraldehyde gave no olefinic products with five different rabbit liver microsomal P450 isozymes. However, increasing activity was seen with the other isomers in the order of isovaleraldehyde, 2-methylbutyraldehyde, and trimethylacetaldehyde, with all of the P450 cytochromes.
PMID:1924356 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC52631 Roberts ES et al; Proc Natl Acad Sci USA 88 (20): 8963-6 (1991)
...inhibits oxidation of other mitochondrial substrates, including acetaldehyde
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5:995
...Isovaleraldehyde is also a degradation product of leucine that is associated with hepatic encephalopathy.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5:995
BUILDING BLOCK

