
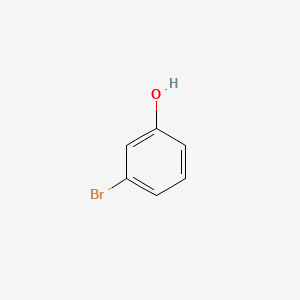

1. Meta-bromophenol
1. 591-20-8
2. M-bromophenol
3. Phenol, 3-bromo-
4. 3-bromo-phenol
5. 3-bromo Phenol
6. Phenol, M-bromo-
7. Mfcd00002253
8. Chembl185651
9. Vmu0x6956y
10. Unii-vmu0x6956y
11. Meta Bromophenol
12. Meta-bromophenol
13. 3-bromanylphenol
14. Hsdb 7649
15. Meta-bromo-phenol
16. B3r
17. Einecs 209-706-5
18. 3-bromophenyl Alcohol
19. 3-bromophenol, 98%
20. Bromophenol, M-
21. 1-bromo-3-hydroxy-benzene
22. M-bromophenol [mi]
23. Schembl49927
24. M-bromophenol [hsdb]
25. Dtxsid9060449
26. Str02996
27. Bdbm50150795
28. Stl280238
29. Akos000118744
30. Ps-4040
31. 3-bromophenol, Purum, >=97.5% (hplc)
32. Am20060304
33. B0629
34. Ft-0601041
35. Ft-0615113
36. En300-19434
37. A832171
38. Q-200364
39. Q26421128
40. F0001-1548
41. Z104473834
42. Inchi=1/c6h5bro/c7-5-2-1-3-6(8)4-5/h1-4,8
43. Phenol, 3-bromo-; Phenol, M-bromo- (8ci); 3-bromophenol; M-bromophenol
| Molecular Weight | 173.01 g/mol |
|---|---|
| Molecular Formula | C6H5BrO |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 20.2 |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 74.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
p-Bromophenol and o-bromophenol were the major urinary phenolic bromobenzene metabolites although m-bromophenol and 4-bromocatechol were also excreted in detectable quantities. With the exception of o-bromophenol, urinary metabolites were excreted primarily as conjugates.
PMID:6710548 Rush GF et al; Toxicology 30 (3): 259-72 (1984)
2-bromophenol (2-(BP)), 3-BP, and 4-BP can all be formed during the metabolism of bromobenzene in both rats and guinea-pigs. 2-BP is formed predominantly by spontaneous isomerization of the 2,3-oxide. 3-BP is formed via the sulfur-series pathway to phenols, which involves the enterohepatic circulation, with the key intermediate being S-(2-hydroxy-4-bromocyclohexa-3,5-dienyl)-L-cysteine, derived from the 4-S-glutathione conjugate of the 3,4-oxide. 4-BP is formed by the sulfur-series route from the S-(2-hydroxy-5-bromocyclohexa-3,5-dienyl)-L-cysteine. Additional suggested in vivo routes to 3- and 4-BP involve dehydration/aromatization of the 3,4-dihydro-3,4-diol, possibly by way of conjugates.
WHO; Concise International Chemical Assessment Document 2,4,6 Tribromophenol and other simple brominated phenols 66 Available from, as of September 24, 2008: https://www.inchem.org/documents/cicads/cicads/cicad66.htm
BUILDING BLOCK

