
1. 4, Vitamin B
2. B 4, Vitamin
3. Vitamin B 4
1. 73-24-5
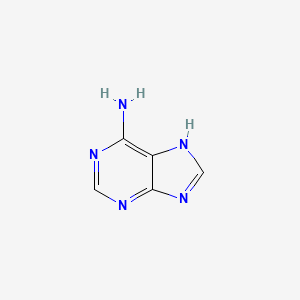
2. 1h-purin-6-amine
3. 6-aminopurine
4. 9h-purin-6-amine
5. 7h-purin-6-amine
6. Vitamin B4
7. Adenin
8. Adeninimine
9. Leuco-4
10. 6-amino-1h-purine
11. 6-amino-3h-purine
12. 6-amino-7h-purine
13. 6-amino-9h-purine
14. 1,6-dihydro-6-iminopurine
15. 3,6-dihydro-6-iminopurine
16. Purine, 6-amino-
17. Usaf Cb-18
18. 1h-purine, 6-amino
19. Adenine [jan]
20. Ade
21. 9h-purine, 1,6-dihydro-6-imino-
22. 1h-purine-6-amine
23. Ccris 2556
24. Ai3-50679
25. 3h-purin-6(7h)-imine
26. Nsc 14666
27. 9h-purine-6-amine
28. 9h-purin-6-ylamine
29. 134434-48-3
30. Chebi:16708
31. 1h-purine, 6-amino-
32. Mfcd00041790
33. Nsc-14666
34. 6h-purin-6-imine, 3,9-dihydro-, (z)- (9ci)
35. Chembl226345
36. 134434-49-4
37. 134454-76-5
38. 66224-66-6
39. Jac85a2161
40. 6h-purin-6-imine, 1,7-dihydro-, (z)- (9ci)
41. 6h-purin-6-imine, 1,9-dihydro-, (e)- (9ci)
42. 6h-purin-6-imine, 3,7-dihydro-, (z)- (9ci)
43. (z)-3,9-dihydro-6h-purin-6-imine
44. 134461-75-9
45. 71660-29-2
46. Ncgc00094856-01
47. Pedatisectine B
48. Dsstox_cid_2557
49. Dsstox_rid_76627
50. Dsstox_gsid_22557
51. Adenine-ring
52. Cas-73-24-5
53. Leucon (tn)
54. Adenine (8ci)
55. Adenine (jan/usp)
56. Adenine [usp:jan]
57. Sr-05000001754
58. 6-aminopurine (adenine)
59. Einecs 200-796-1
60. 1h-purin-6(9h)-imine
61. 1h-purin-6-amine (9ci)
62. 3h-purin-6-amine (9ci)
63. Unii-jac85a2161
64. 3h-adenine
65. 6-amino Purine
66. 6-amino-purine
67. Purin-6-amine
68. 1jys
69. 1nli
70. 1wei
71. 2pqj
72. 3kpv
73. [3h]adenine
74. Adenine, 1
75. Adenine,(s)
76. Albb-005925
77. 7h-purin-6-ylamine
78. 71660-30-5
79. (s)-norfluoxetine-d5
80. 9h-purin-6-yl-amin
81. Adenine-[15n2]
82. Spectrum_001106
83. 2p8n
84. Starbld0001134
85. 9h-purin-6-yl-amine
86. Adenine [vandf]
87. Adenine-[8-13c]
88. Specplus_000535
89. Adenine [inci]
90. Adenine, >=99%
91. 9h-purin-6-amine #
92. Adenine [mi]
93. Adenine [mart.]
94. Spectrum2_000583
95. Spectrum3_000616
96. Spectrum4_001891
97. Spectrum5_000542
98. Adenine [usp-rs]
99. Adenine [who-dd]
100. 6-aminopurine;vitamin B4
101. Bmse000060
102. Bmse000861
103. Bmse000995
104. Epitope Id:140097
105. Adenine, Cell Culture Grade
106. Schembl8110
107. Oprea1_057274
108. Us9138393, Adenine
109. Us9144538, Adenine
110. Bspbio_002152
111. Kbiogr_002447
112. Kbiogr_002562
113. Kbioss_001586
114. Kbioss_002571
115. Zinc882
116. Mls001066342
117. Divk1c_006631
118. Spectrum1500807
119. Spbio_000426
120. Adenine [ep Monograph]
121. Adenine [usp Impurity]
122. Gtpl4788
123. Adenine [usp Monograph]
124. 9h-purine,6-dihydro-6-imino-
125. Dtxsid6022557
126. Bdbm33218
127. Kbio1_001575
128. Kbio2_001586
129. Kbio2_002562
130. Kbio2_004154
131. Kbio2_005130
132. Kbio2_006722
133. Kbio2_007698
134. Kbio3_001652
135. Kbio3_003040
136. 1,9-dihydro-6h-purin-6-imine
137. Adenine 100 Microg/ml In Water
138. Cmap_000085
139. 7h-purin-6-amine, Min. 95%
140. Bcpp000433
141. Bdbm181146
142. Hms1921i14
143. Hms2092k20
144. Hms2269i04
145. Pharmakon1600-01500807
146. Bcp02865
147. Hy-b0152
148. Nsc14666
149. Vca70030
150. Tox21_111348
151. Tox21_302108
152. Bbl007925
153. Ccg-38506
154. Nsc757793
155. S1981
156. Stk387542
157. Wln: T56 Bm Dn Fn Hnj Iz
158. Akos000118903
159. Akos005171607
160. Tox21_111348_1
161. Ac-2028
162. Am83908
163. Bcp9000233
164. Cs-1984
165. Db00173
166. Nsc-757793
167. Sdccgmls-0066584.p001
168. Ncgc00094856-02
169. Ncgc00094856-03
170. Ncgc00094856-05
171. Ncgc00255120-01
172. 1217770-71-2
173. As-11841
174. Bl008313
175. Nci60_000998
176. Smr000471871
177. Adenosine Impurity A [ep Impurity]
178. Sbi-0052324.p002
179. Adenine, Vetec(tm) Reagent Grade, >=99%
180. Db-013503
181. A0149
182. Ft-0620943
183. Ft-0656198
184. 73a245
185. Adenine, Suitable For Cell Culture, Bioreagent
186. C00147
187. D00034
188. P50008
189. Q15277
190. Z-1043
191. Ab00052833-18
192. Ab00052833-19
193. Ab00052833_20
194. Ab00052833_22
195. Ab00052833_23
196. Ab00052833_24
197. A935233
198. Q-200595
199. Sr-05000001754-1
200. Sr-05000001754-2
201. W-106856
202. Adenine, Bioreagent, Plant Cell Culture Tested, >=99%
203. Adenine, European Pharmacopoeia (ep) Reference Standard
204. F0001-1848
205. Z1250132272
206. 6379c0e0-c1bb-4087-96c5-1de281b8ea4c
207. Adenine, United States Pharmacopeia (usp) Reference Standard
208. Adenine, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 135.13 g/mol |
|---|---|
| Molecular Formula | C5H5N5 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 135.05449518 g/mol |
| Monoisotopic Mass | 135.05449518 g/mol |
| Topological Polar Surface Area | 80.5 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 127 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For nutritional supplementation, also for treating dietary shortage or imbalance
Adenine (sometimes known as vitamin B4) combines with the sugar ribose to form adenosine, which in turn can be bonded with from one to three phosphoric acid units, yielding AMP, ADP and ATP . These adenine derivatives perform important functions in cellular metabolism. Adenine is one of four nitrogenous bases utilized in the synthesis of nucleic acids. A modified form of adenosine monophosphate (cyclic AMP) is an imporant secondary messenger in the propagation of many hormonal stimuli. Adenine is an integral part of the structure of many coenzymes. Adenosine (adenine with a ribose group) causes transient heart block in the AV node of the heart. In individuals suspected of suffering from a supraventricular tachycardia (SVT), adenosine is used to help identify the rhythm. Certain SVTs can be successfully terminated with adenosine.
Adenine forms adenosine, a nucleoside, when attached to ribose, and deoxyadenosine when attached to deoxyribose, and it forms adenosine triphosphate (ATP), which drives many cellular metabolic processes by transferring chemical energy between reactions.
BUILDING BLOCK

