




1. 75143-89-4
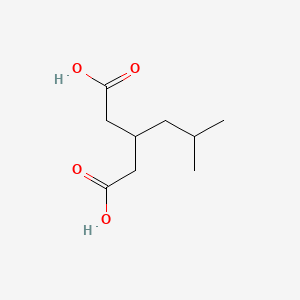
2. 3-(2-methylpropyl)pentanedioic Acid
3. 3-isobutylpentanedioic Acid
4. Pentanedioic Acid, 3-(2-methylpropyl)-
5. Isobutylglutaric Acid
6. Emh1ivr5qh
7. 3-isobutyl Glutaric Acid
8. Mfcd15529751
9. 3-isobutylglutaricacid
10. Pregabalin Impurity 4
11. Unii-emh1ivr5qh
12. Schembl1410027
13. Amy4101
14. Dtxsid00450420
15. Bcp12370
16. Zinc49588169
17. Akos015841726
18. Cs-w019327
19. Ac-25625
20. As-10103
21. Sy033309
22. Ft-0657207
23. D70576
24. 143i894
25. A838334
26. J-512713
27. 2,3-dihydro-1h-indole-4-carbonitrilehydrochloride
28. 3-(2-methylpropyl)pentanedioic Acid (3-isobutylglutaric Acid)
| Molecular Weight | 188.22 g/mol |
|---|---|
| Molecular Formula | C9H16O4 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 188.10485899 g/mol |
| Monoisotopic Mass | 188.10485899 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 170 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




