
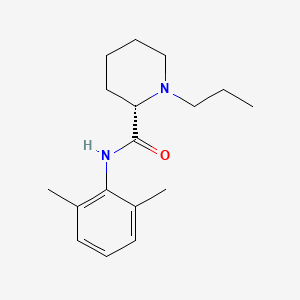

1. 1 Propyl 2',6' Pipecoloxylidide
2. 1-propyl-2',6'-pipecoloxylidide
3. Al 381
4. Al-381
5. Al381
6. Lea 103
7. Lea-103
8. Lea103
9. Naropeine
10. Naropin
11. Ropivacaine Hydrochloride
12. Ropivacaine Monohydrochloride
13. Ropivacaine Monohydrochloride, (s)-isomer
1. 84057-95-4
2. (s)-ropivacaine
3. Naropin
4. Ropivacaine [inn]
5. Ropivacaine Hydrochloride
6. Ropivacaina
7. Ropivacainum
8. Ropivacaina [spanish]
9. (s)-n-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide
10. Ropivacainum [inn-latin]
11. Ropivacaina [inn-spanish]
12. (2s)-n-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide
13. Ropivicaine
14. 7io5lya57n
15. (-)-1-propyl-2',6'-pipecoloxylidide
16. Lea 103
17. Chebi:8890
18. Tlc590
19. (2s)-n-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide
20. (2s)-n-(2,6-dimethylphenyl)-1-propyl-piperidine-2-carboxamide
21. Tlc-590
22. Ropivacaine (inn)
23. L-n-n-propylpipecolic Acid-2,6-xylidide
24. Lea-103
25. 2-piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, (2s)-
26. S-ropivacaine
27. (s)-(-)-1-propyl-2',6'-pipecoloxylidide
28. 1-propyl-2',6'-pipecoloxylidide
29. Noropine
30. Narop
31. Lea-103 Hcl
32. Naropin (tn)
33. Ncgc00164597-01
34. Ropivacaine [inn:ban]
35. 98717-15-8
36. Unii-7io5lya57n
37. Ropivacaine Base
38. Mfcd00864425
39. Al-381
40. Ropivacaine [mi]
41. Ropivacaine [vandf]
42. Schembl33292
43. Ropivacaine [usp-rs]
44. Ropivacaine [who-dd]
45. Bidd:gt0203
46. Narop; Noropine; Lea-103
47. Gtpl7602
48. (-)-1-propyl-2',6'-dimethyl-2-piperidylcarboxyanilid
49. Chembl1077896
50. Dtxsid4040187
51. Zinc897002
52. Hy-b0563
53. Bbl102321
54. S5504
55. Stl556120
56. Akos017343283
57. Ccg-267197
58. Db00296
59. As-35173
60. Cs-0009514
61. R0251
62. C07532
63. D08490
64. Ab00698466-07
65. Ab00698466_10
66. 057r954
67. A840710
68. Q279504
69. (s)-(-)-1-propyl-2',6'-pipecoloxylidine
70. Q-201677
71. (-)-1-propyl-2',6'-dimethyl-2-piperidylcarboxyanilide
72. (s)-n-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide
73. (s)-(-)-1-propylpiperidine-2-carboxylic Acid (2,6-dimethylphenyl)amide
| Molecular Weight | 274.4 g/mol |
|---|---|
| Molecular Formula | C17H26N2O |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 274.204513457 g/mol |
| Monoisotopic Mass | 274.204513457 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 308 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in obstetric anesthesia and regional anesthesia for surgery.
FDA Label
Ropivacaine, a local anesthetic agent, is indicated for the production of local or regional anesthesia or analgesia for surgery, for oral surgery procedures, for diagnostic and therapeutic procedures, and for obstetrical procedures.
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
N01BB09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BB - Amides
N01BB09 - Ropivacaine
Absorption
Bioavailability is 87%98% following epidural administration.
Route of Elimination
Ropivacaine is extensively metabolized in the liver, predominantly by aromatic hydroxylation mediated by cytochrome P4501A to 3-hydroxy ropivacaine. After a single IV dose approximately 37% of the total dose is excreted in the urine as both free and conjugated 3-hydroxy ropivacaine. In total, 86% of the ropivacaine dose is excreted in the urine after intravenous administration of which only 1% relates to unchanged drug.
Clearance
387+/- 107 mL/min
unbound plasma clearance=7.2 +/- 1.6 L/min
Hepatic
Ropivacaine has known human metabolites that include 3-hydroxy-ropivacaine and PPX.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Approximately 4.2 hours.
Local anesthetics such as Ropivacaine block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. Specifically, they block the sodium-channel and decrease chances of depolarization and consequent action potentials. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers.
BUILDING BLOCK

