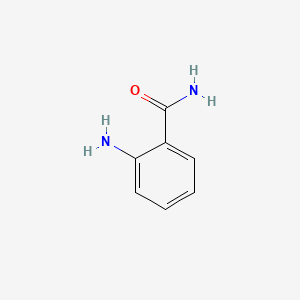

1. Anthranilamide
2. Ortho-aminobenzamide
1. Anthranilamide
2. 88-68-6
3. Benzamide, 2-amino-
4. O-aminobenzamide
5. 2-carbamoylaniline
6. Aminobenzamide
7. Anthranilimidic Acid
8. Anthranilic Acid Amide
9. Benzamide, O-amino-
10. 2-amino-benzamide
11. Anthranilamide (van)
12. O-aminobenzamide (van)
13. 2-aminobenzamide (van)
14. Anthranilimidic Acid (van)
15. Benzoic Acid, 2-amino-, Amide
16. 28144-70-9
17. Mfcd00007981
18. Benzamide, O-amino- (van)
19. 2-aminobenzimidic Acid
20. Q1m2wek6va
21. Nsc-38768
22. Hsdb 5261
23. Benzenecarboximidicacid, 2-amino-
24. Einecs 201-851-2
25. Unii-q1m2wek6va
26. Nsc 38768
27. Brn 0508509
28. Amino-benzamide
29. Ai3-28018
30. O-carbamoylaniline
31. Oxytocin-d5
32. O-amino-benzamide
33. 2-aminobenz-amide
34. 2-amino Benzamide
35. Ortho-aminobenzamide
36. 2- Amino-benzamide
37. 2-amino Benzamide, 7
38. 2-aminobenzenecarboxamide
39. Anthranilamide, >=98%
40. Dsstox_cid_1789
41. Aminobenzamide, 2-
42. Dsstox_rid_76327
43. Dsstox_gsid_21789
44. Oprea1_246280
45. Schembl38228
46. 4-14-00-01010 (beilstein Handbook Reference)
47. Mls001066328
48. Chembl43175
49. 2-amino-benzamideanthranilamide
50. 2-aminobenzamide [hsdb]
51. Dtxsid2021789
52. Schembl11068162
53. Bdbm33219
54. Chebi:193638
55. Hms1732d11
56. Hms2269g08
57. Zinc152578
58. Cs-d1680
59. Nsc38768
60. Str02027
61. Tox21_200412
62. Bbl012278
63. Stl163592
64. Benzamide, O-amino- (van) (8ci)
65. Akos000119694
66. Ps-5040
67. Cas-88-68-6
68. Discontinued. Please See O878507
69. 2-carbamoylaniline, Anthranilimidic Acid
70. Ncgc00247015-01
71. Ncgc00247015-02
72. Ncgc00257966-01
73. Ac-20346
74. Smr000112353
75. Sy001144
76. Db-022491
77. A0262
78. Ft-0611226
79. D71115
80. 2-aminobenzamide 100 Microg/ml In Acetonitrile
81. A842842
82. At-057/40177776
83. W-100391
84. Q27286892
85. Z57036632
86. F3329-0460
87. Anthranilamide, Matrix Substance For Maldi-ms, >=99.0% (hplc)
88. 2ae
| Molecular Weight | 136.15 g/mol |
|---|---|
| Molecular Formula | C7H8N2O |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 136.063662883 g/mol |
| Monoisotopic Mass | 136.063662883 g/mol |
| Topological Polar Surface Area | 69.1 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 136 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fluorescent Dyes
Chemicals that emit light after excitation by light. The wave length of the emitted light is usually longer than that of the incident light. Fluorochromes are substances that cause fluorescence in other substances, i.e., dyes used to mark or label other compounds with fluorescent tags. (See all compounds classified as Fluorescent Dyes.)
Recent experiments with administration of anthranilic acid in rats afforded two new metabolites; anthranilamide and a light blue fluorescent substance. Administration of anthranilamide to rats resulted in urinary excretion of two main metabolites which were isolated from urine as 3-hydroxyanthranilamide-O-sulfate and 5-hydroxyanthranilamide-O-sulfate. This fact showed that anthranilamide is hydroxylated and then conjugated in vivo. Hydroxylation of anthranilamide was found to be catalyzed by drugs hydroxylation system but not by the microsomes alone, and is a specific reaction activated by a protein component contained in the soluble fraction. The purified protein component was recognized that it was bluish green protein containing copper and zinc, and its molecular weight was 25,700. This protein component had no effect on the metabolism of drugs by liver microsomes but showed specific activation of hydroxylation of 2-aminobenzoyl compounds.
PMID:1244107 Ishiguro I et al; Acta Vitaminol Enzymol 29 (6): 278-82 (1975)
ADP-ribose synthesis inhibitor
PMID:2147666 Kuykendall JR et al; Differentiation 44 (1): 69-73 (1990)
MARKET PLACE



LOOKING FOR A SUPPLIER?