




1. 881995-70-6
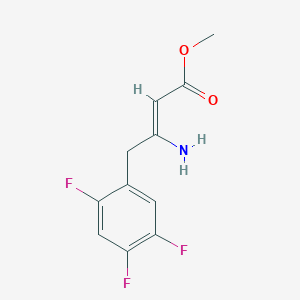
2. Methyl (z)-3-amino-4-(2,4,5-trifluorophenyl)but-2-enoate
3. Schembl742800
4. Schembl2861837
5. Amy10656
6. Zinc95496246
7. Akos030525017
8. Cs-0165434
9. (z)-methyl3-amino-4-(2,4,5-trifluorophenyl)but-2-enoate
10. Methyl(z)-3-amino-4-(2,4,5-trifluorophenyl)but-2-enoate
11. (z)-methyl-3-amino-4-(2,4,5-trifluorophenyl)but-2-enoate
12. (z)-3-amino-4-(2,4,5-trifluorophenyl)-2-butenoic Acid Methyl Ester
13. (z)-methyl 3-amino-4-(2,4,5-trifluorophenyl)but-2-enoate? (sitagliptin Impurity Pound(c)
14. 1402237-72-2
| Molecular Weight | 245.20 g/mol |
|---|---|
| Molecular Formula | C11H10F3NO2 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 52.3 |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 309 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |








