

1. 5 Aminosalicylate
2. 5 Aminosalicylic Acid
3. 5-aminosalicylate
4. 5-aminosalicylic Acid
5. Asacol
6. Asacolon
7. Ascolitin
8. Canasa
9. Claversal
10. Fivasa
11. Hydrochloride, Mesalamine
12. Lixacol
13. M Aminosalicylic Acid
14. M-aminosalicylic Acid
15. Mesalamine Hydrochloride
16. Mesalamine Monosodium Salt
17. Mesalazine
18. Mesasal
19. Meta Aminosalicylic Acid
20. Meta-aminosalicylic Acid
21. Monosodium Salt, Mesalamine
22. Novo 5 Asa
23. Novo-5 Asa
24. Pentasa
25. Rowasa
26. Salofalk
1. 5-aminosalicylic Acid
2. Mesalazine
3. 89-57-6
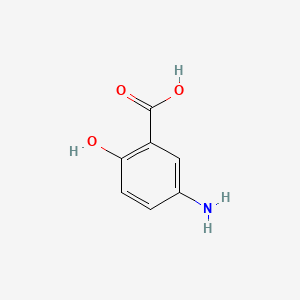
4. 5-amino-2-hydroxybenzoic Acid
5. Asacol
6. Pentasa
7. Canasa
8. 5-asa
9. Claversal
10. Rowasa
11. Salofalk
12. M-aminosalicylic Acid
13. Lialda
14. Mesasal
15. Benzoic Acid, 5-amino-2-hydroxy-
16. Fisalamine
17. Apriso
18. Lixacol
19. Sfrowasa
20. P-aminosalicylsaeure
21. Asacolitin
22. Mesalazina
23. Mesalazinum
24. Iialda
25. 5-amino-2-hydroxy-benzoic Acid
26. Asacol Hd
27. Mesalamine [usan]
28. 5-amino Salicylic Acid
29. Salicylic Acid, 5-amino-
30. 2-hydroxy-5-aminobenzoic Acid
31. Max-002
32. 3-carboxy-4-hydroxyaniline
33. Mfcd00007877
34. Nsc 38877
35. Nsc-38877
36. Mesalazine [inn]
37. Mls001424012
38. 4q81i59gxc
39. Chebi:6775
40. Mesalamine (usan)
41. 5-?aminosalicylic Acid (mesalazine)
42. Cas-89-57-6
43. Ncgc00016344-03
44. Mesalazinum [latin]
45. Smr000145728
46. Dsstox_cid_4506
47. Dsstox_rid_77435
48. Dsstox_gsid_24506
49. Mesalazina [spanish]
50. P-aminosalicylsaeure [german]
51. Mesavancol
52. Delzicol
53. Mesavance
54. Mezavant
55. Mesalazine Mmx
56. Mezavant Xl
57. Mesalamine (usp)
58. Pentasa (tn)
59. Salofalk Granu-stix
60. Apriso (tn)
61. Asacol (tn)
62. Canasa (tn)
63. Lialda (tn)
64. Rowasa (tn)
65. 5-as
66. Ccris 7334
67. Sr-01000763486
68. Mesalamine [usan:usp]
69. Einecs 201-919-1
70. Brn 2090421
71. Unii-4q81i59gxc
72. Ai3-15564
73. Hsdb 7512
74. Ajg-501
75. Spd 476
76. Spd-476
77. Spd-480
78. Mesalamine (tn)
79. Delzicol (tn)
80. Sfrowasa (tn)
81. Mesalamine (lialda)
82. 5-aminosalicylic_acid
83. Md-0901
84. 5-aminosalicyclic Acid
85. 5-amino-salicylic Acid
86. Mesalamine [mi]
87. Mesalazine [jan]
88. Prestwick0_001069
89. Prestwick1_001069
90. Prestwick2_001069
91. Prestwick3_001069
92. Mesalamine [hsdb]
93. Wln: Zr Dq Cvq
94. Z-206
95. Mesalamine [vandf]
96. Chembl704
97. Mesalazine (jp17/inn)
98. Ec 201-919-1
99. Cid_4075
100. Mesalazine [mart.]
101. Mesalamine [usp-rs]
102. Mesalazine [who-dd]
103. Oprea1_847633
104. Schembl31297
105. 3amino-6-hydroxybenzoic Acid
106. Bspbio_001058
107. Kbiogr_002425
108. Kbioss_002431
109. 4-14-00-02058 (beilstein Handbook Reference)
110. Mls000758287
111. 5-aminosalicylic Acid, 95%
112. 5-aminosalicylic Acid, Tablet
113. Bidd:gt0811
114. 3-amino-6-hydroxybenzoic Acid
115. Spbio_002969
116. Bpbio1_001164
117. Gtpl2700
118. Zinc1688
119. 5-amino 2-hydroxy Benzoic Acid
120. Dtxsid5024506
121. Mesalamine [orange Book]
122. Mesalazine [ep Impurity]
123. Schembl18038934
124. 5-aminosalicylic Acid, >=99%
125. Bdbm60918
126. Kbio2_002425
127. Kbio2_004993
128. Kbio2_007561
129. Kbio3_002904
130. Mesalazine [ep Monograph]
131. Cmap_000045
132. Hms1571e20
133. Hms2051m21
134. Hms2090i09
135. Hms2098e20
136. Hms3393m21
137. Hms3649k15
138. Hms3651m15
139. Hms3715e20
140. Mesalamine [usp Monograph]
141. Pharmakon1600-01505993
142. Bcp05326
143. Nsc38877
144. Tox21_110384
145. Tox21_201610
146. Tox21_303125
147. Ac8101
148. Bbl013046
149. Nsc759301
150. S1681
151. Stk301678
152. Akos000118959
153. Tox21_110384_1
154. Ac-2764
155. Bcp9000175
156. Ccg-100829
157. Db00244
158. Hs-0100
159. Nc00079
160. Nsc-759301
161. Ncgc00016344-01
162. Ncgc00016344-02
163. Ncgc00016344-04
164. Ncgc00016344-05
165. Ncgc00016344-07
166. Ncgc00090934-01
167. Ncgc00090934-02
168. Ncgc00257142-01
169. Ncgc00259159-01
170. 5-amino-2-hydroxobenzoic Acid Monohydrate
171. Bp-13074
172. Hy-15027
173. Sy002854
174. 5-aminosalicylic Acid, Analytical Standard
175. A0317
176. Ab00374979
177. Am20060091
178. Ft-0619950
179. Sw197303-4
180. C07138
181. D00377
182. Ab00374979-09
183. Ab00374979-10
184. Ab00374979_11
185. Ab00374979_12
186. Q412479
187. 5-amino-2-hydroxybenzoic Acid,5-aminosalicylic Acid
188. Q-201355
189. Sr-01000763486-3
190. Sr-01000763486-4
191. Sr-01000763486-9
192. Z57127471
193. F1918-0003
194. Mesalazine, European Pharmacopoeia (ep) Reference Standard
195. Mesalamine, United States Pharmacopeia (usp) Reference Standard
196. Mesalamine, Pharmaceutical Secondary Standard; Certified Reference Material
197. Mesalazine For System Suitability, European Pharmacopoeia (ep) Reference Standard
198. 51481-17-5
| Molecular Weight | 153.14 g/mol |
|---|---|
| Molecular Formula | C7H7NO3 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 153.042593085 g/mol |
| Monoisotopic Mass | 153.042593085 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mesalamine rectal suspension is indicated for the treatment of mild to moderate distal ulcerative colitis, proctosigmoiditis, and proctitis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Mesalamine rectal suspension is indicated to help maintain remission of distal ulcerative colitis. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Mesalamine suppositories are indicated for the treatment of active ulcerative proctitis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Mesalamine is indicated to treat and to maintain remission of mild to moderate ulcerative colitis or (Crohn's disease). /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1931
Lialda /the first oral once daily mesalamine tablet/ is indicated for the induction of remission in patients with active, mild to moderate ulcerative colitis.
FDA: Center for Drug Evaluation and Research (CDER); Label Information for Lialda (Mesalamine) Lasted updated 2007. Available from, as of March 30, 2007: https://www.fda.gov/cder/foi/label/2007/022000lbl.pdf
Oral and rectal mesalamine preparations usually are well tolerated. The most common adverse effects of oral or rectal mesalamine are GI effects and headache. In clinical studies, most adverse effects associated with oral or rectal preparations were mild in severity and were transient or reversible. However, adverse effects have been severe enough to require discontinuance of the drug in less than 1% or in up to about 4-5% of patients receiving rectal or oral mesalamine, respectively, although in some studies, the rate of discontinuance of the drug was similar to or less than in those receiving placebo. Most of the adverse effects reported with the use of oral mesalamine delayed-release tablets were similar in short- and long-term studies.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3013
Exacerbation of colitis symptoms was reported in 3% of patients receiving oral mesalamine delayed-release tablets. Other adverse GI effects associated with oral mesalamine extended-release capsules and occurring in less than 1% of patients, include abdominal distention, constipation, duodenal ulcer, dysphagia, eructation, esophageal or mouth ulcer, fecal incontinence, GI bleeding (e.g., rectal bleeding), stool abnormalities (e.g., change in color or texture), oral moniliasis, and thirst, although a causal relationship to the drug of many of these adverse effects has not been established.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3013
In controlled clinical trials in patients receiving oral mesalamine delayed-release tablets, abdominal pain, eructation, nausea, diarrhea, dyspepsia, vomiting, constipation, flatulence, exacerbation of colitis, abdominal enlargement, gastroenteritis, GI hemorrhage, rectal disorder (e.g., hemorrhage, tenesmus), and stool abnormalities, were the most common adverse GI effects, occurring in about 2-18% of patients; dry mouth, indigestion, stomatitis, and cramping were reported rarely. Frequency of these GI effects did not seem to increase with increased dosages, although in uncontrolled studies, the incidence of abdominal pain, flatulence, and GI bleeding were dose related. The most common adverse GI effects of oral mesalamine extended-released capsules were diarrhea (including melena), nausea, abdominal pain, dyspepsia, vomiting, anorexia, worsening of ulcerative colitis, and rectal urgency, occurring in greater than 0.4-3% of patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3013
An acute intolerance syndrome (sensitivity reaction), characterized by cramping, abdominal pain, bloody diarrhea, and, occasionally, fever, headache, malaise, conjunctivitis, pruritus, and rash, has occurred in a few patients receiving mesalamine and required prompt discontinuance of the drug. In patients manifesting such intolerance, a history of sulfasalazine intolerance, if any, should be reevaluated.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3014
For more Drug Warnings (Complete) data for MESALAMINE (26 total), please visit the HSDB record page.
Mesalazine is indicated for the induction of remission in patients with active or mild to moderate acute exacerbations of ulcerative colitis and for the maintenance of remission of ulcerative colitis,. Prescribing information for mesalazine in the UK also indicates the medication for the maintenance of remission of Crohn's ileo-colitis.
FDA Label
Mesalazine is one of the two components of sulphasalazine, the other being sulphapyridine. It is the latter which is responsible for the majority of the side effects associated with sulphasalazine therapy whilst mesalazine is known to be the active moiety in the treatment of ulcerative colitis. The pharmacodynamic actions of mesalazine occur in the colonic/rectal mucosae local to the delivery of drug from mesalazine tablets into the lumen. There is information suggesting that the severity of colonic inflammation in ulcerative colitis patients treated with mesalazine is inversely correlated with mucosal concentrations of mesalazine. Plasma concentrations representing systemically absorbed mesalazine are not believed to contribute extensively to efficacy.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
A07EC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07E - Intestinal antiinflammatory agents
A07EC - Aminosalicylic acid and similar agents
A07EC02 - Mesalazine
Absorption
Depending on the formulation administered, prescribing information for orally administered delayed-released tablets of 2.4g or 4.8g of mesalazine given once daily for 14 days to healthy volunteers was to found to be about 21% to 22% of the administered dose while prescribing information for an orally administered controlled-release capsule formulation suggests 20% to 30% of the mesalazine in the formulation is absorbed. In contrast, when mesalamine is administered orally as an unformulated 1-g aqueous suspension, mesalazine is approximately 80% absorbed.
Route of Elimination
Elimination of mesalazine is mainly via the renal route following metabolism to N-acetyl-5-aminosalicylic acid (acetylation). However, there is also limited excretion of the parent mesalazine drug in the urine. After the oral administration of the extended-release formulation of mesalazine, of the approximately 21% to 22% of the drug absorbed, less than 8% of the dose was excreted unchanged in the urine after 24 hours, compared with greater than 13% for N-acetyl-5-aminosalicylic acid. When given the controlled-release formulation, about 130 mg free mesalazine was recovered in the feces following a single 1-g dose, which was comparable to the 140 mg of mesalazine recovered from the molar equivalent sulfasalazine tablet dose of 2.5 g F3001]. Elimination of free mesalazine and salicylates in feces increased proportionately with the dose given. N-acetylmesalazine was the primary compound excreted in the urine (19% to 30%) following the controlled-release dosing.
Volume of Distribution
The apparent volume of distribution (Vd) of the drug in adults is approximately 0.2 L/kg.
Clearance
The mean (SD) renal clearance in L/h for mesalazine following the single dose administration of mesalazine delayed-release tablets 4.8g under fasting conditions to young and elderly subjects was documented as 2.05 (1.33) in young subjects aged 18 to 35 years old, 2.04 (1.16) in elderly subjects aged 65 to 75 years old, and 2.13 (1.20) in elderly subjects older than 75 years.
Following oral administration, low concentrations of mesalamine and higher concentrations of its metabolite, N-acetyl-5-aminosalicylic acid, have been detected in human breast milk. It is not known whether mesalamine or its metabolites are distributed into milk in humans following rectal administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
Mesalamine and N-acetyl-5-aminosalicylic acid cross the placenta following oral administration; however, serum concentrations of mesalamine in umbilical cord and amniotic fluid are very low. It is not known whether mesalamine crosses the placenta following rectal administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
In vitro, mesalamine and N-acetyl-5-aminosalicylic acid are approximately 44-55 and 80% bound, respectively, to plasma proteins. Protein binding of N-acetyl-5-aminosalicylic acid does not appear to be concentration dependent at concentrations ranging from 1-10 ug/mL.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
It is generally accepted that 5-aminosalicylate (5-ASA; mesalamine), widely used in inflammatory bowel disease therapy, exerts its action from the intraluminal site of the intestine. In addition to local metabolism of 5-ASA, it has been assumed that therapeutic mucosal concentrations of 5-ASA depend on transporter-mediated secretion back to the lumen. ... The hypothesis that 5-ASA represents a substrate of P-glycoprotein (P-gp) and/or multidrug resistance-associated protein 2 (MRP2), thereby possibly contributing to variable therapeutic effects /was tested/. Polarized, basal-to-apical transport of [(3)H]5-ASA was studied in monolayers of Caco-2 and L-MDR cells, both of which express P-gp in their apical membrane, as well as in MDCK cells transfected with human MRP2. Moreover, we investigated the influence of 5-ASA on transport of digoxin in Caco-2 cells. In Caco-2 cells a P-gp-mediated efflux of 5-ASA (5-500 muM) could be excluded. Likewise, in L-MDR1 and MRP2 cells no transport differences in either the basal-to-apical or apical-to-basal direction were measurable. 5-ASA (50 muM to 5 mM) had no effect on the transport of digoxin. ...
PMID:16944117 Xin HW et al; Eur J Clin Pharmacol 62 (10): 871-875 (2006)
For more Absorption, Distribution and Excretion (Complete) data for MESALAMINE (15 total), please visit the HSDB record page.
The primary metabolite of mesalazine (5-aminosalicylic acid) is predominantly N-acetyl-5-aminosalicylic acid (Ac-5-ASA). This metabolite is generated via N-acetyltransferase (NAT) activity in the liver and intestinal mucosa cells, largely by NAT-1, in particular.
Mesalazine (5-aminosalicylic acid, 5-ASA), an anti-inflammatory agent for the treatment of inflammatory bowel diseases, is metabolized in organism to the principal biotransformation product, N-acetyl-5-ASA. Some other phase II metabolites (N-formyl-5-ASA, N-butyryl-5-ASA, N-beta-d-glucopyranosyl-5-ASA) have also been described. 5-ASA is a polar compound and besides it exhibits amphoteric properties. ...
PMID:16466733 Nobilis M et al; J Chromatogr A 1119 (1-2): 299-308 (2006)
The exact metabolic fate of mesalamine has not been clearly established. The drug undergoes rapid N-acetylation, probably in the liver, to form N-acetyl-5-aminosalicylic acid; mesalamine and N-acetyl-5-aminosalicylic acid also may undergo conjugation with glucuronic acid. Several other, unidentified metabolites also may be formed. It has been suggested that N-acetylation also may occur (to a limited extent) in the intestinal wall and/or the lumen. The intestinal flora probably are involved in this acetylation, and extensive floral acetylation may adversely affect clinical efficacy of the drug. Correlation between acetylator phenotype of patients receiving mesalamine and the degree of N-acetylation does not appear to exist. Although it has been suggested that N-acetyl-5-aminosalicylic acid may be pharmacologically active, therapeutic response has been poor in some patients treated rectally with this metabolite, and the relative contribution of this metabolite to the therapeutic effect of mesalamine remains questionable. N-Acetyl-5-aminosalicylic acid did not inhibit lipoxygenase in vitro.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3017
Mesalazine has known human metabolites that include mesalazine, N-acetyl.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The apparent elimination half-life documented for oral delayed-release mesalazine tablets is 7 to 12 hours. The elimination half-life recorded for the active N-acetyl-5-aminosalicylic acid metabolite generated from the administration of oral delayed-release mesalazine tablets is 12 to 23 hours.
Elimination of metabolite: 5 to 10 hr /N-acetyl-5-aminosalicylci acid/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Elimination: 0.5-1.5 hours
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1934
Although the mechanism of action of mesalazine is not fully understood, it is believed to possess a topical anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalazine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon. Furthermore, mesalazine also has the potential to inhibit the activation of Nuclear Factor kappa B (NKkB) and consequently the production of key of pro-inflammatory cytokines. It has been proposed that reduced expression of PPAR gamma nuclear receptors (gamma form of peroxisome proliferator-activated receptors) may be implicated in ulcerative colitis. There is evidence that mesalazine produces pharmacodynamic effects through direct activation of PPAR gamma receptors in the colonic/rectal epithelium as well. Moreover, since increased leukocyte migration, abnormal cytokine production, increased production of arachidonic acid metabolites, particularly leukotriene B4, and increased free radical formation in the inflamed intestinal tissue are all present in patients with inflammatory bowel disease it is also believed that mesalazine has in-vitro and in-vivo pharmacological effects that inhibit leukocyte chemotaxis, decrease cytokine and leukotriene production and scavenge for free radicals.
Mesalamine is effective in the treatment of inflammatory bowel diseases. However, the mechanisms of action of mesalamine remain unclear. IEC-6 and IRD-98, nontransformed rat small intestinal epithelial cell lines, were used to examine the effect of mesalamine on the expression of manganese superoxide dismutase (MnSOD). Rats were given mesalamine enemas to determine the effect on colonic MnSOD expression. Treatment with mesalamine at 0.02 or 2 mg/mL induced MnSOD mRNA levels 2.67-fold or 5.66-fold, respectively. Inhibition of 5-lipoxygenase activating protein with MK-886 or cyclooxygenase with indomethacin did not influence the level of MnSOD mRNA. Nuclear run-on experiments demonstrated an increase in de novo transcription following treatment with mesalamine. MnSOD protein levels were induced 2-fold at 24 hr and 4.23-fold at 48 hr following treatment with 1 mg/mL mesalamine. Mesalamine increased MnSOD 1.7-fold in vivo. Pretreatment with mesalamine significantly protected IRD-98 cells from tumor necrosis factor-alpha cytotoxicity. This is the first example of transcriptional gene regulation by mesalamine. The induction of MnSOD by mesalamine may contribute to the therapeutic mechanism of mesalamine.
PMID:11557525 Valentine JF; Am J Physiol Gastrointest Liver Physiol 281 (4): G1044-50 (2001)
Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with inflammatory bowel disease. Mesalamine appears to diminish inflammation by inhibiting cyclooxygenase and lipoxygenase, thereby decreasing the production of prostaglandins, and leukotrienes and hydroxyeicosatetraenoic acids (HETs), respectively. It is also believed that mesalamine acts aas a scavenger of oxygen-derived free radicals, which are produced in greater numbers in patients with inflammatory bowel disease.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1931
Derivatives of 5-aminosalicylic acid (mesalamine) represent a mainstay in inflammatory bowel disease therapy, yet the precise mechanism of their therapeutic action is unknown. Because tumor necrosis factor (TNF)-alpha is important in the pathogenesis of inflammatory bowel disease, we investigated the effect of mesalamine on TNF-alpha-regulated signal transduction and proliferation in intestinal epithelial cells. Young adult mouse colon cells were studied with TNF-alpha, epidermal growth factor, or ceramide in the presence or absence of mesalamine. Proliferation was studied by hemocytometry. Mitogen-activated protein (MAP) kinase activation and IkappaBalpha expression were determined by Western blot analysis. Nuclear transcription factor kappaB (NF-kappaB) nuclear translocation was determined by confocal laser immunofluorescent microscopy. The antiproliferative effects of TNF-alpha were blocked by mesalamine. TNF-alpha and ceramide activation of MAP kinase were inhibited by mesalamine, whereas epidermal growth factor activation of MAP kinase was unaffected. TNF-alpha-stimulated NF-kappaB activation and nuclear translocation and the degradation of Ikappa-Balpha were blocked by mesalamine. Mesalamine inhibits TNF-alpha-mediated effects on intestinal epithelial cell proliferation and activation of MAP kinase and NF-kappaB. Therefore, it may function as a therapeutic agent based on its ability to disrupt critical signal transduction events in the intestinal cell necessary for perpetuation of the chronic inflammatory state.
PMID:10029619 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3606885 Kaiser GC et al; Gastroenterology 116 (3): 602-9 (1999)
BUILDING BLOCK

CAS Number : 89-57-6
End Use API :
End Use API : Mesalazine
About the Company : BEC Chemicals Pvt. Ltd., was founded by Late Mr. M. T. Shah in 1979 and is specialized in manufacturing of Active pharmaceutical Ingredients (API’s) and Intermediates. The company’s...

CAS Number : 89-57-6
End Use API :
End Use API : Sulfasalazine
About the Company : SMS Lifesciences is a global player in APIs/Intermediates manufacturing having a strong research and manufacturing team supported by state of the art facilities. What started off as a s...

Zhejiang Hengkang Pharm Group is a dynamic pharmaceutical entity, spanning drug research, large-scale production, and global marketing.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
IOL Chemicals is an innovation-driven bulk drug, Intermediate and Specialty Chemicals company.
HRV Global Life Sciences - Market Expansion Leader in Pharmaceuticals.
Cambrex is an innovative life sciences company with a refreshingly human approach.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Midd...
HRV Global Life Sciences - Market Expansion Leader in Pharmaceuticals.
