

1. 911705-40-3
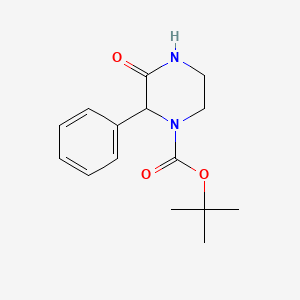
2. 3-oxo-2-phenyl-piperazine-1-carboxylic Acid Tert-butyl Ester
3. Schembl2539220
4. 1-boc-3-oxo-2-phenylpiperazine
5. Xwyogpgttfwzap-uhfffaoysa-n
6. Cs-d0486
7. Mfcd28579819
8. Akos027255478
9. As-68179
10. W10309
11. Tert-butyl3-oxo-2-phenylpiperazine-1-carboxylate
| Molecular Weight | 276.33 g/mol |
|---|---|
| Molecular Formula | C15H20N2O3 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 58.6 |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 370 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
BUILDING BLOCK

