



1. 915416-45-4
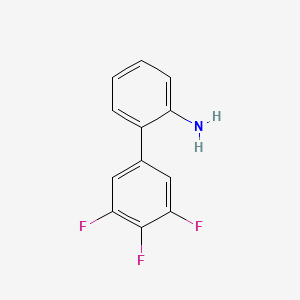
2. 3',4',5'-trifluoro-[1,1'-biphenyl]-2-amine
3. 3',4',5'-trifluorobiphenyl-2-amine
4. 3',4',5'-trifluorobiphenyl-2-ylamine
5. 3,4,5-trifluoro-2'-aminobiphenyl
6. [1,1'-biphenyl]-2-amine, 3',4',5'-trifluoro-
7. 10xda3w53s
8. 3',4',5'-trifluoro(1,1'-biphenyl)-2-amine
9. (1,1'-biphenyl)-2-amine, 3',4',5'-trifluoro-
10. 3',4',5'-trifluoro[1,1'-biphenyl]-2-amine
11. Unii-10xda3w53s
12. Schembl397483
13. Schembl977502
14. Dtxsid60658041
15. Bcp11782
16. Mfcd14603436
17. Zinc40770693
18. 3',4',5'-trifluoro-2-aminobiphenyl
19. Akos010489403
20. Sb79864
21. Ds-18225
22. Cs-0156689
23. C77129
24. 3',4',5'-trifluoro[1,1'-biphenyl]-2-yl-amine
25. A851907
26. Q27251166
27. Z1318516286
| Molecular Weight | 223.19 g/mol |
|---|---|
| Molecular Formula | C12H8F3N |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 223.06088375 g/mol |
| Monoisotopic Mass | 223.06088375 g/mol |
| Topological Polar Surface Area | 26 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 224 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



