
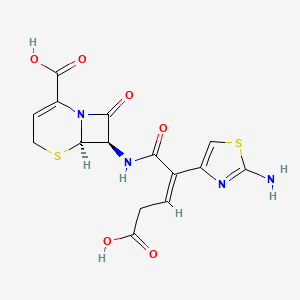

1. 7-(2-(2-amino-4-thiazolyl)-4-carboxy-2-butenoylamino)-3-cephem-4-carboxylic Acid
2. 7432 S
3. 7432-s
4. Cedax
5. Sch 39720
6. Sch-39720
7. Sch39720
1. 97519-39-6
2. Cedax
3. Ceftibuteno
4. Ceftibutenum
5. Ceftibutene
6. Sch 39720
7. Ceftibuten Hydrate
8. Cis-ceftibuten
9. Sch-39720
10. 7432-s
11. Antibiotic 7432s
12. Nsc-758925
13. Achn383
14. Achn-383
15. Chebi:3510
16. Iw71n46b4y
17. (+)-(6r,7r)-7-((z)-2-(2-amino-4-thiazolyl)-4-carboxycrotonamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
18. Ceftibuten Dihydrate
19. Cis-ceftibutin
20. 97519-39-6 (free)
21. Isocef
22. Cetb
23. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((2-(2-amino-4-thiazolyl)-4-carboxy-1-oxo-2-butenyl)amino)-8-oxo-, (6r-(6alpha,7beta(z)))-
24. Cephalosporin 7432-s
25. (6r,7r)-7-[[(z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
26. (6r,7r)-7-((z)-2-(2-aminothiazol-4-yl)-4-carboxybut-2-enamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
27. Ceftibutene [inn-french]
28. Ceftibutenum [inn-latin]
29. Ceftibuteno [inn-spanish]
30. Unii-iw71n46b4y
31. Ceftibutin
32. Ceprifran
33. Ceftem
34. Keimax
35. Ceftibuten [usan:inn:ban]
36. S 7432
37. Ncgc00095137-01
38. Ceftibuten-13c3
39. Ceftibuten, 95%
40. Mfcd00864918
41. Ceftibuten [mi]
42. Ceftibuten [inn]
43. Ceftibuten (usan/inn)
44. Spectrum5_001558
45. Ceftibuten [usan]
46. Ceftibuten [vandf]
47. Ceftibutin [vandf]
48. Ceftibuten [who-dd]
49. Chembl1605
50. Dsstox_cid_25925
51. Dsstox_rid_81227
52. Dsstox_gsid_45925
53. Schembl37054
54. Bspbio_002733
55. Spectrum1505207
56. Dtxsid4045925
57. Gtpl12029
58. Hms1922l17
59. Hms2093k18
60. Hms3715p10
61. Pharmakon1600-01505207
62. Hy-b0698
63. Zinc3871967
64. Tox21_111446
65. Bdbm50370586
66. Ccg-39440
67. Nsc758925
68. Akos005146205
69. Akos015854930
70. Db01415
71. Nsc 758925
72. Ncgc00178501-01
73. Ncgc00178501-04
74. Sbi-0206740.p001
75. Cas-97519-39-6
76. C-2550
77. C08117
78. D00922
79. Ab01563048_01
80. 519c396
81. Ceftibuten, Antibiotic For Culture Media Use Only
82. Q419521
83. Sr-05000001989
84. Sr-05000001989-1
85. 7-[2-(2-amino-1,3-thiazol-4-yl)-4-carboxyisocrotonamide]-3-cephem-4-carboxylicacid
86. (6r,7r)-7-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
87. (6r,7r)-7-[[(2z)-2-(2-amino-4-thiazolyl)-4-carboxy-1-oxo-2-buten-1-yl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
88. (6r,7r)-7-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
89. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((2-(2-amino-4-thiazolyl)-4-carboxy-1-oxo-2-butenyl)amino)-8-oxo-, (6r-(6.alpha.,7.beta.(z)))-
90. 7beta-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino}-3,4-didehydrocepham-4-carboxylic Acid
| Molecular Weight | 410.4 g/mol |
|---|---|
| Molecular Formula | C15H14N4O6S2 |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 410.03547653 g/mol |
| Monoisotopic Mass | 410.03547653 g/mol |
| Topological Polar Surface Area | 217 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 755 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of acute bacterial exacerbations of chronic bronchitis (ABECB), acute bacterial otitis media, pharyngitis, and tonsilitis.
Ceftibuten is an antibiotic with bactericidal actions.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DD - Third-generation cephalosporins
J01DD14 - Ceftibuten
Absorption
Rapidly absorbed following oral administration.
Route of Elimination
Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces.
Volume of Distribution
0.21 L/kg [adult subjects]
0.5 L/kg [fasting pediatric patients]
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer is approximately 1/8 as antimicrobially potent as the cis-isomer.
Ceftibuten exerts its bactericidal action by binding to essential target proteins of the bacterial cell wall. This binding leads to inhibition of cell-wall synthesis.
BUILDING BLOCK



LOOKING FOR A SUPPLIER?