




1. 429659-01-8
2. Dabigatran Impurity 14
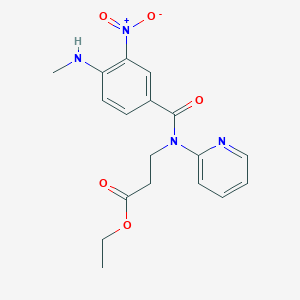
3. Ethyl 3-[[4-(methylamino)-3-nitrobenzoyl](pyridin-2-yl)amino]propanoate
4. Ethyl 3-[[4-(methylamino)-3-nitrobenzoyl]-pyridin-2-ylamino]propanoate
5. Ethyl3-(4-(methylamino)-3-nitro-n-(pyridin-2-yl)benzamido)propanoate
6. Ethyl 3-(4-(methylamino)-3-nitro-n-(pyridin-2-yl)benzamido) Propanoate
7. Ethyl N-[4-(methylamino)-3-nitrobenzoyl]-n-pyridin-2-yl-ss-alaninate
8. Schembl327138
9. Dtxsid501025573
10. Bcp12900
11. Cs-m2455
12. Mfcd09833623
13. Zinc22011559
14. Akos015924422
15. Ac-7904
16. As-72447
17. Db-004037
18. J-520801
19. Ethyl 3-(4-(methylamino)-3-nitro-n-(pyridin-2yl)benzamido)propanoate
20. N-[4-(methylamino)-3-nitrobenzoyl]-n-(2-pyridyl)-beta-alanine Ethyl Ester
21. Ethyl 3-{1-[4-(methylamino)-3-nitrophenyl]-n-(pyridin-2-yl)formamido}propanoate
| Molecular Weight | 372.4 g/mol |
|---|---|
| Molecular Formula | C18H20N4O5 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 372.14336975 g/mol |
| Monoisotopic Mass | 372.14336975 g/mol |
| Topological Polar Surface Area | 117 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 524 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |

















