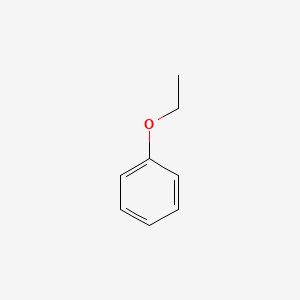

1. Ethoxybenzene
2. Ethyl Phenyl Ether
1. Ethoxybenzene
2. 103-73-1
3. Benzene, Ethoxy-
4. Ethyl Phenyl Ether
5. Phenyl Ethyl Ether
6. Phenetol
7. Benzene, Ethoxy
8. Phenoxyethane
9. Nsc 406706
10. Ether, Ethyl Phenyl-
11. Nsc-406706
12. Rb8lu2c57f
13. Chebi:67129
14. Nsc406706
15. A Phenoxyethane
16. Ether, Ethyl Phenyl
17. Phenylcthylether
18. Ethoxybenzene; Ethyl Phenyl Ether; Nsc 406706; Phenetol; Phenoxyethane; Phenyl Ethyl Ether
19. Hsdb 112
20. Einecs 203-139-7
21. Mfcd00009090
22. Unii-rb8lu2c57f
23. Ethoxy-benzene
24. Methyl Anisole
25. Ai3-05616
26. 1-ethoxybenzene
27. Ethoxybenzene, 99%
28. Phenetole [mi]
29. Phenetole [hsdb]
30. Ec 203-139-7
31. Wln: 2or
32. Schembl18492
33. Chembl499585
34. Dtxsid7059278
35. Zinc1599383
36. Stl282470
37. Akos000120160
38. Pb47848
39. Ls-13425
40. Nsc406706nsc 406706
41. Cs-0017190
42. E0043
43. Ft-0656867
44. D78866
45. A800791
46. Q419340
47. J-001025
48. F1908-0060
| Molecular Weight | 122.16 g/mol |
|---|---|
| Molecular Formula | C8H10O |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 122.073164938 g/mol |
| Monoisotopic Mass | 122.073164938 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 65 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... EXCRETED AS GLUCURONIDE & ETHEREAL SULFATE.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2525
... PHENETOLE, LIKE ANISOLE, IS HYDROXYLATED IN PARA POSITION & EXCRETED AS GLUCURONIDE & ETHEREAL SULFATE.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2525
Wild type, mutant, and recombinant bacterial strains capable of oxidizing aromatic hydrocarbons were screened for their ability to oxidize anisole, and phenetole. Toluene induced cells of Pseudomonas putida F39/D transformed anisole to a compound tentatively identified as cis-1,2-dihydroxy-3-methoxycyclohexa-3,5-diene, 2-methoxyphenol, catechol, and trace amounts of phenol while phenetole was converted primarily to cis-1,2-dihydroxy-3-ethoxycyclohexa-3,5-diene and 2-ethoxyphenol. Induced cells of Pseudomonas sp. NCIB 9816/11 and Beijerinckia sp. B8/36 transformed anisole to phenol, and phenetole to phenol and ethenyloxybenzene. Toluene induced cells of P. putida BG1 converted anisole to phenol but did not oxidize phenetole. In contrast, toluene induced cells of P. mendocina KRl, which oxidize toluene via monooxygenation at the para position, transformed anisole to 4-methoxyphenol, and phenetole to 2-, 3-and 4-ethoxyphenol. The involvement of toluene and naphthalene dioxygenases in the reactions catalyzed by strains F39/D and NCIB 9816/11, respectively, was confirmed with recombinant E. coli strains expressing the cloned dioxygenase genes. The results show that the oxygenases from different Pseudomonas strains oxidize anisole and phenetole to different hydroxylated products.
Resnick SM, Gibson DT; Biodegradation 4 (3): 195-203 (1993)
BUILDING BLOCK

