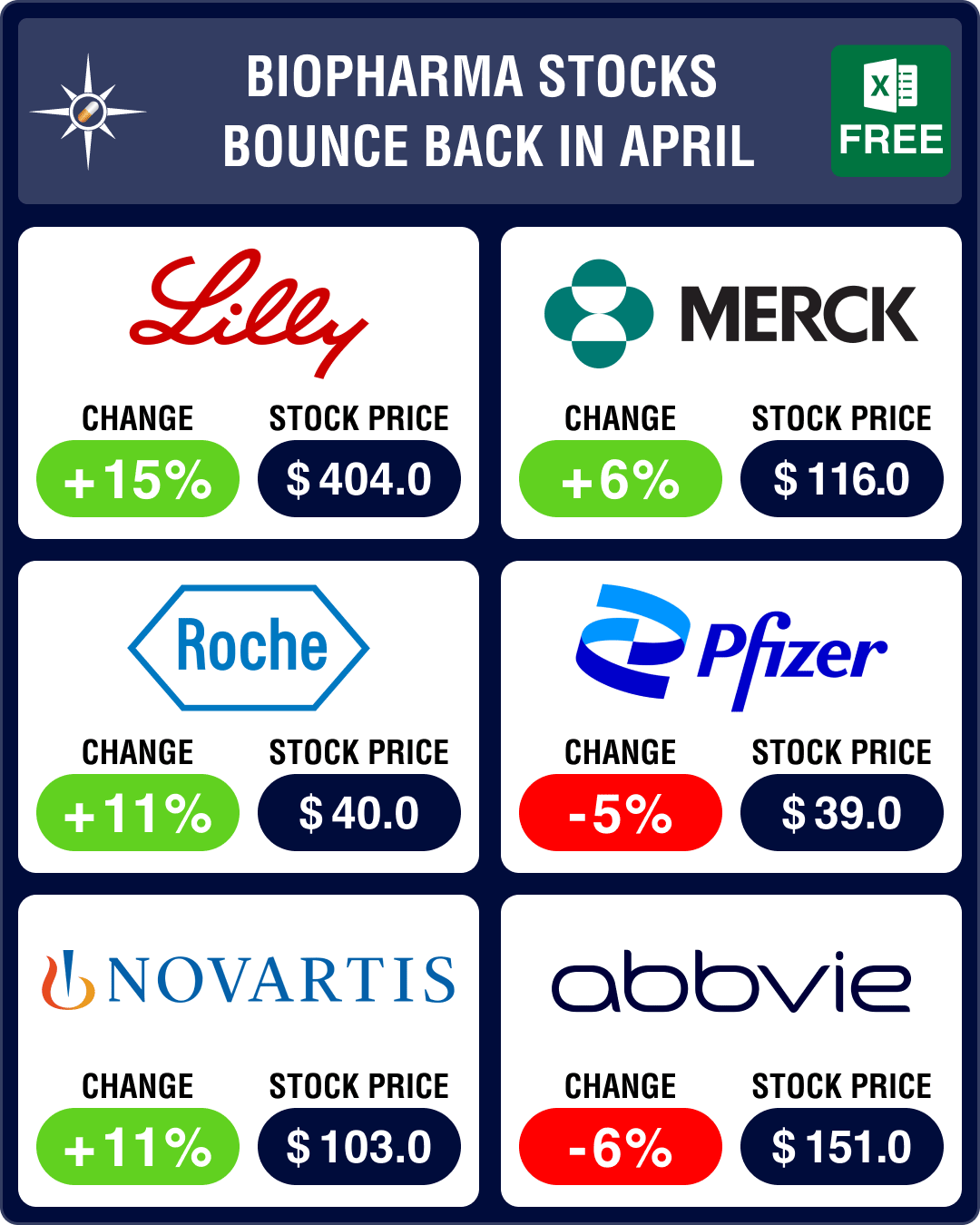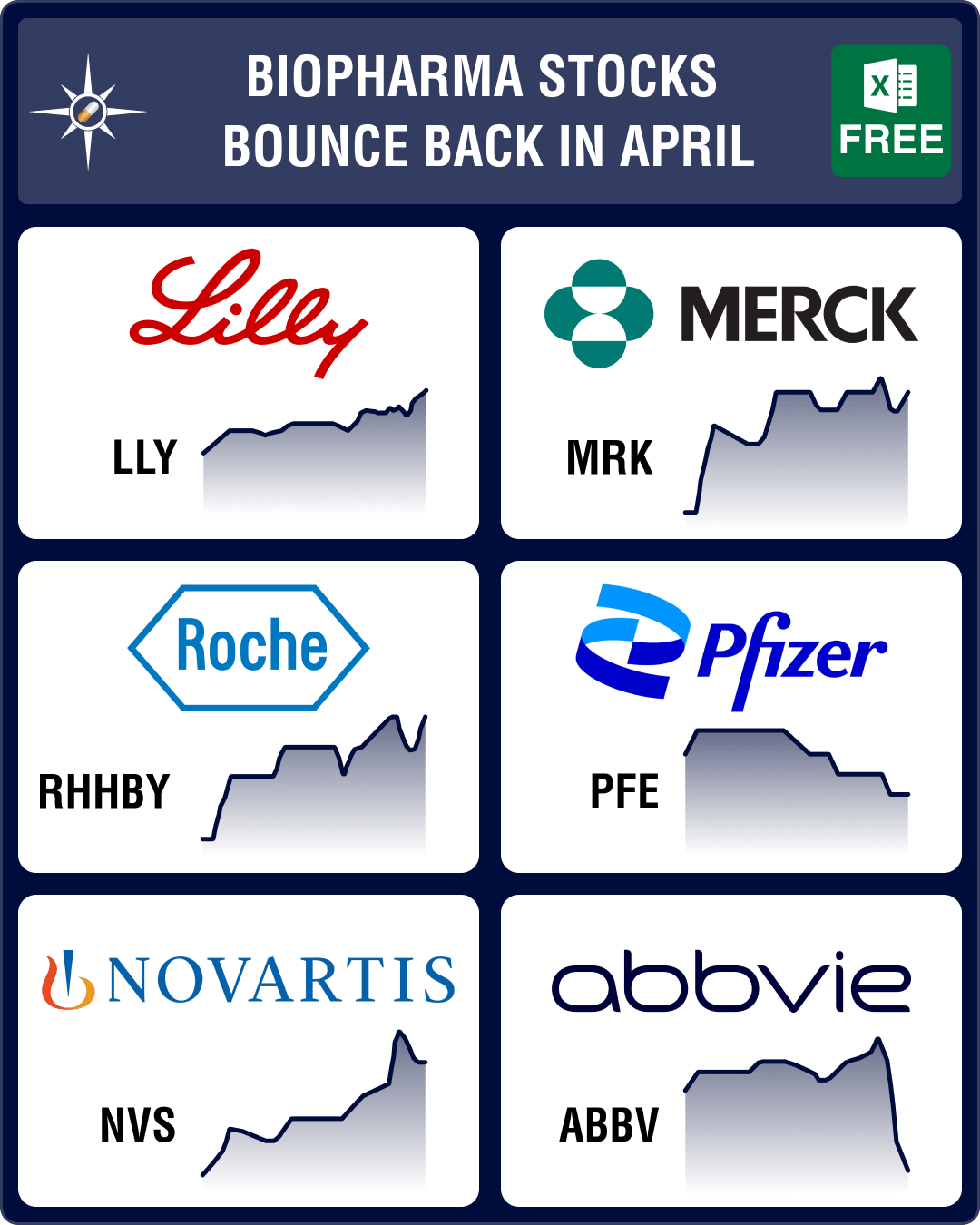
Stock Recap #PipelineProspector
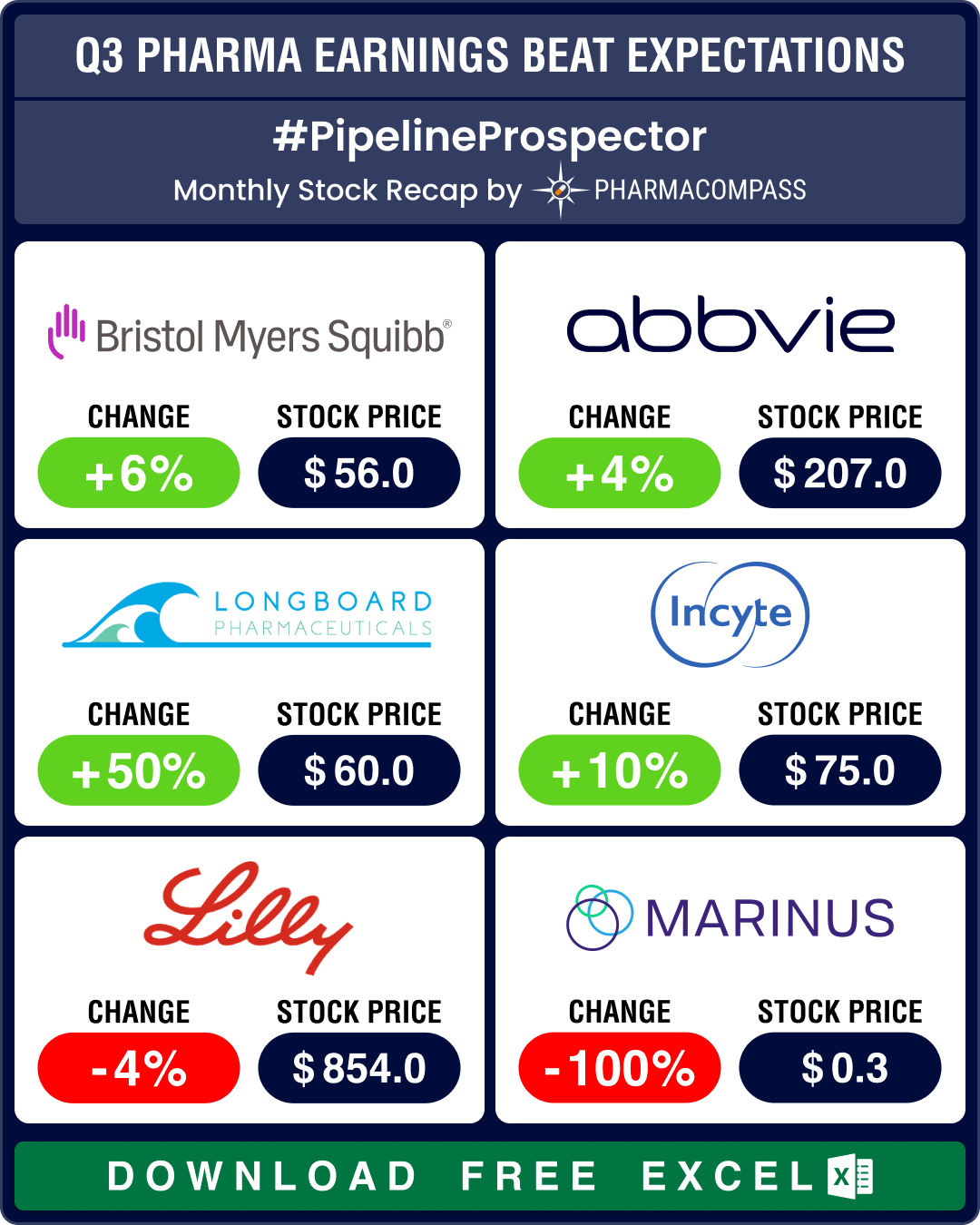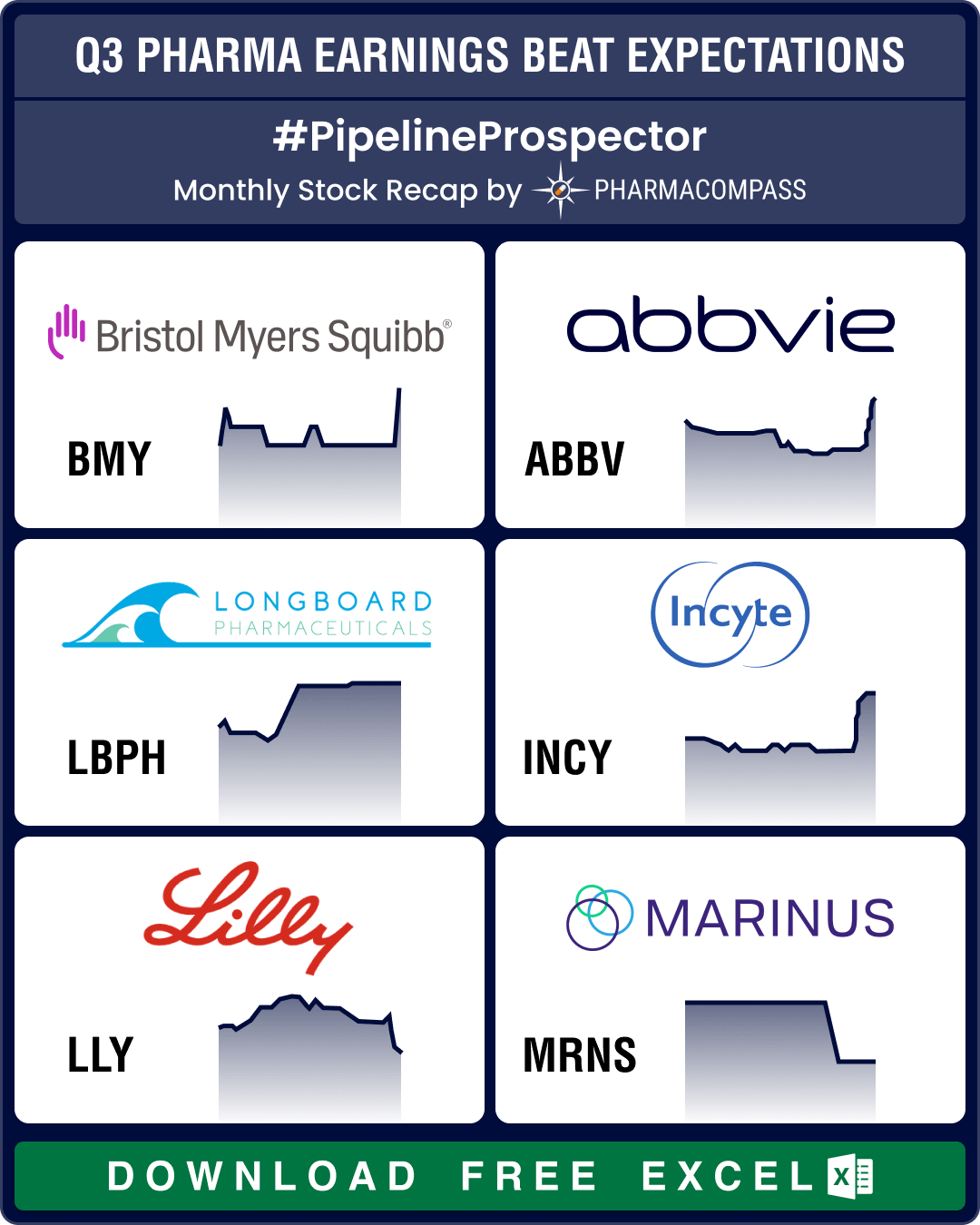
Pipeline Prospector Oct 2024: Lundbeck acquires Longboard for US$ 2.6 bn; molecular glue degrader tech witnesses dealmaking
In October, several pharma companies posted their third quarter (Q3) results. Drugmakers like Pfizer
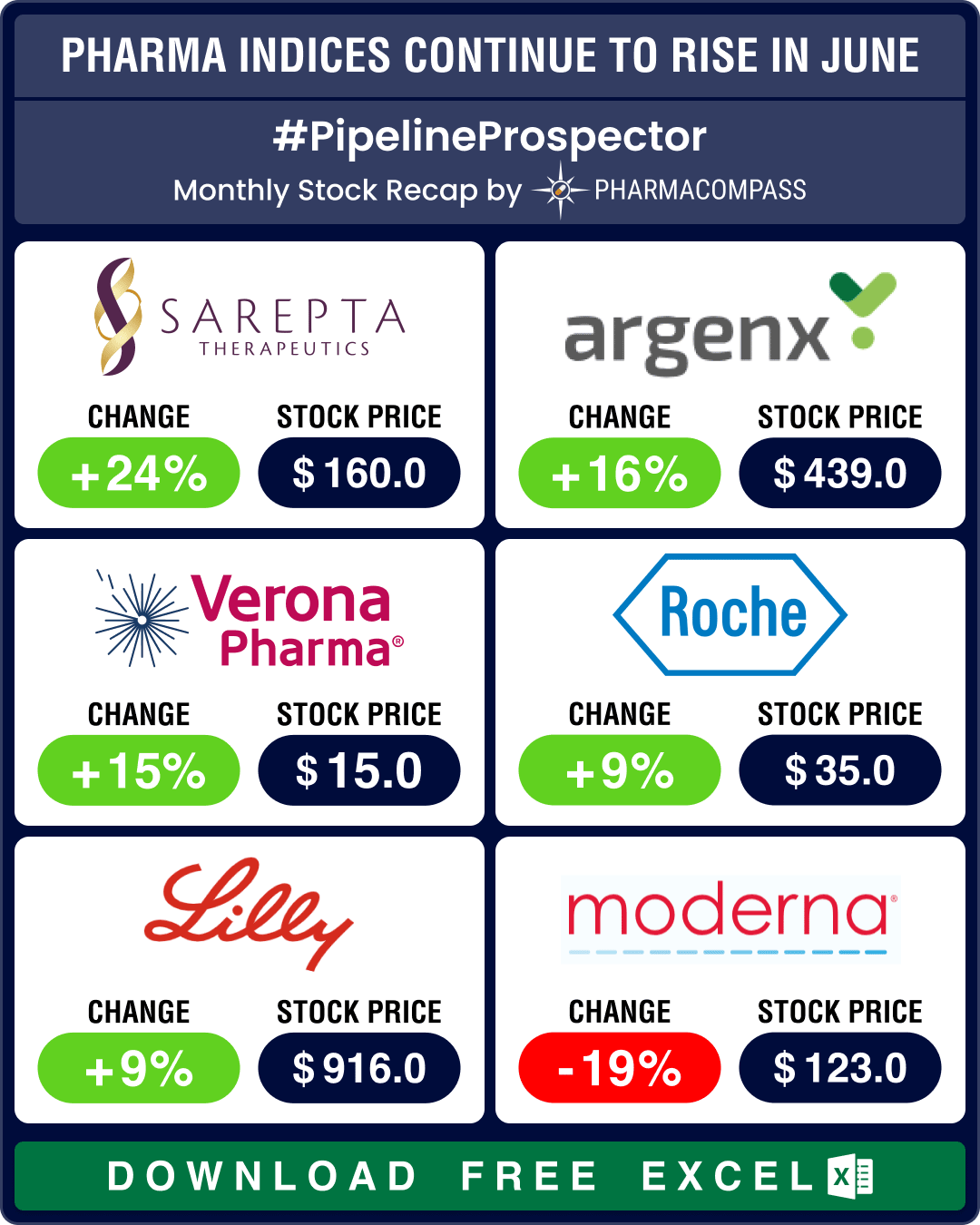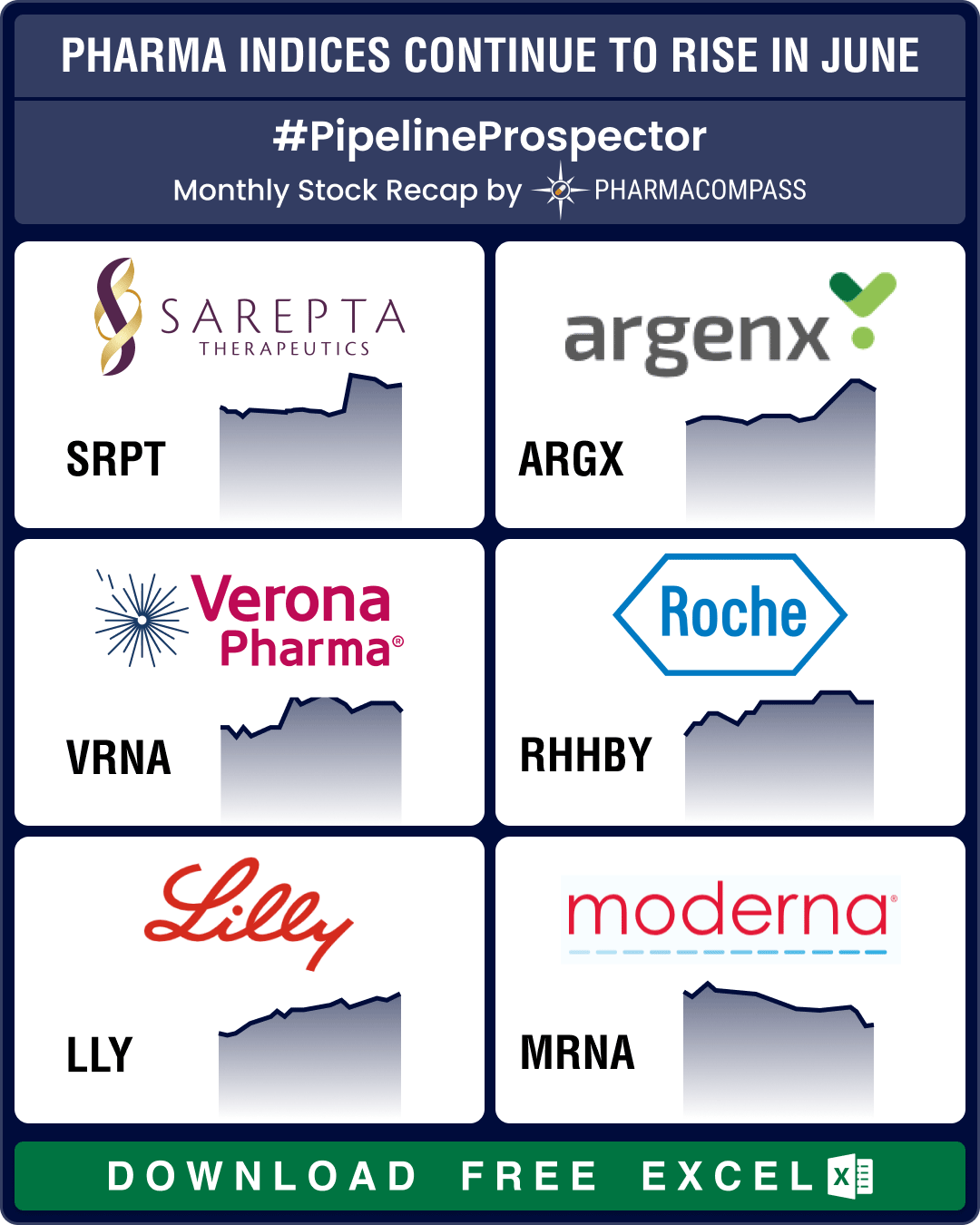
Pipeline Prospector June 2024: FDA approves Merck’s next-gen pneumococcal vaccine, Verona’s COPD therapy
The pharma indices were back in the black in May, and the good streak continued through June with th
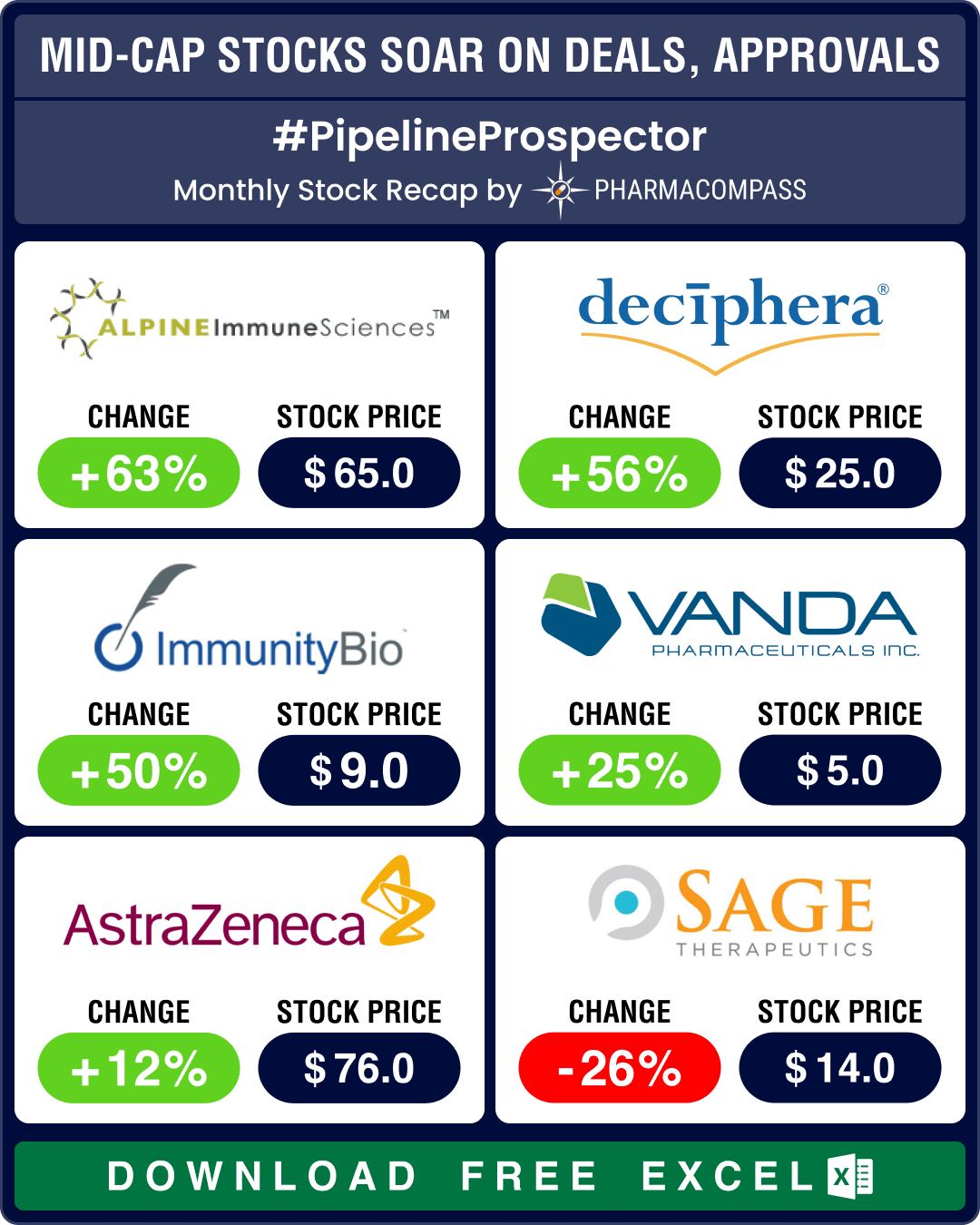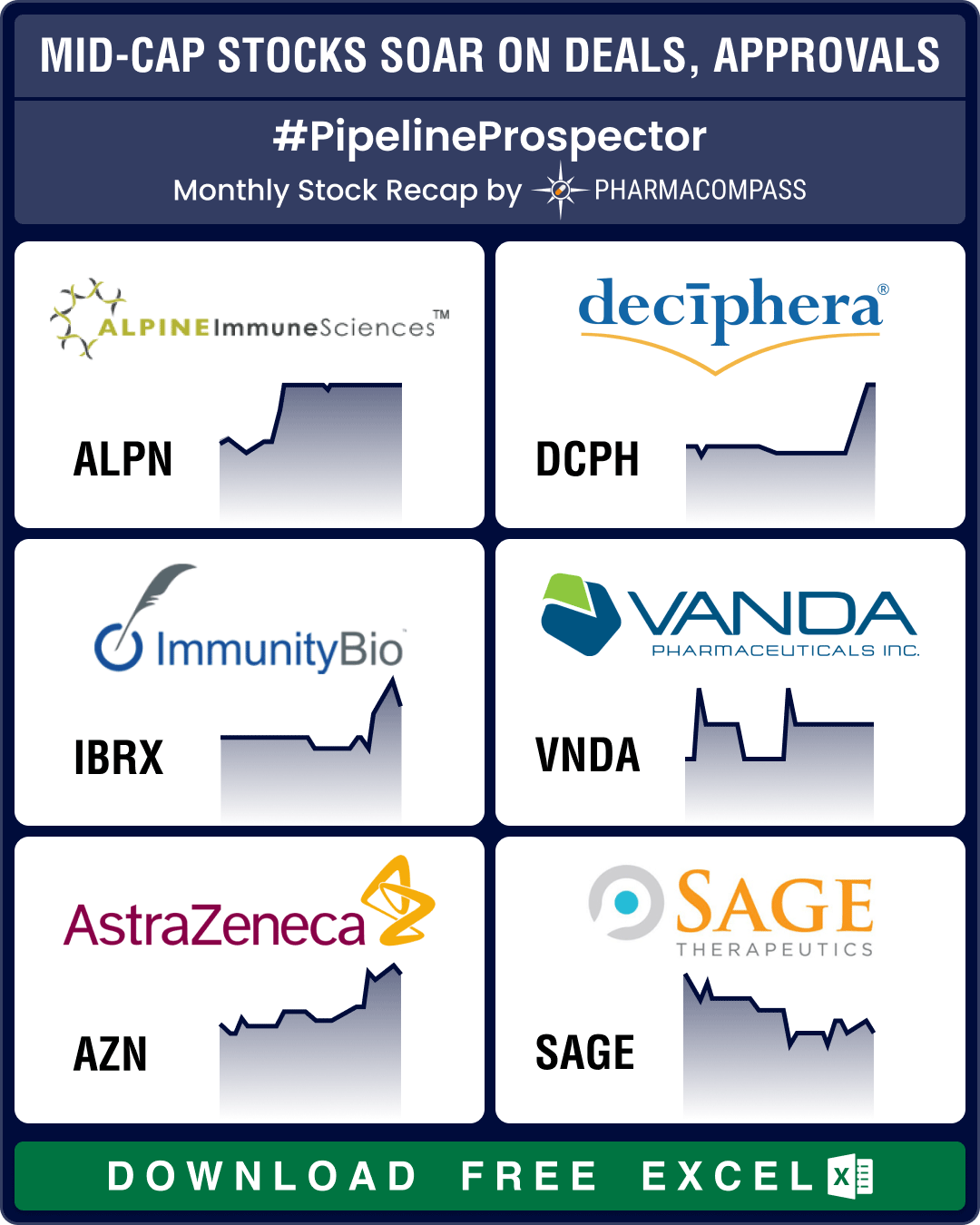
Pipeline Prospector April 2024: Indices dip amid muted Q1 results; Vertex acquires Alpine Immune for US$ 4.9 bn
Pharma indices had begun to recede in March. Their red streak accelerated in April with the Nasdaq B
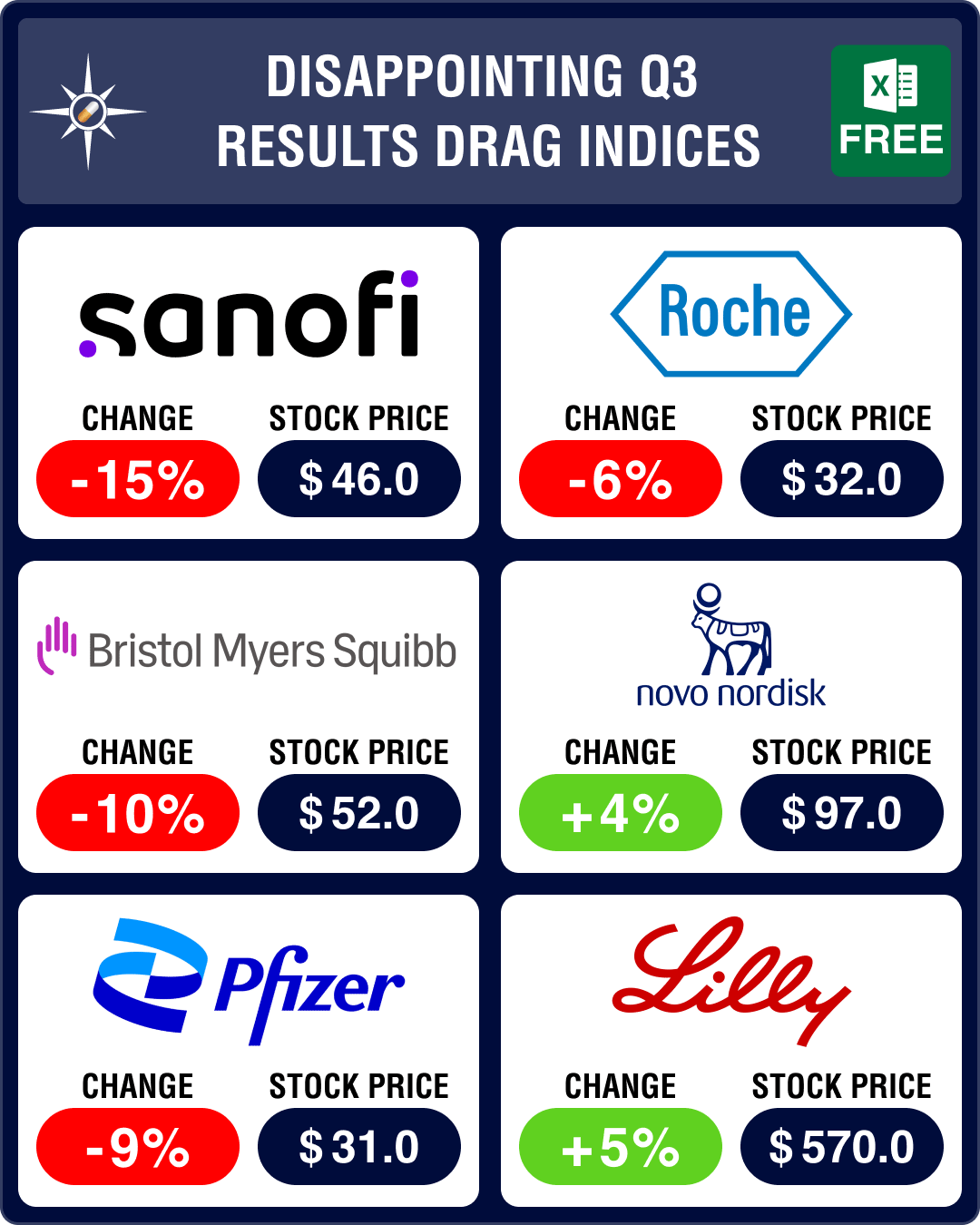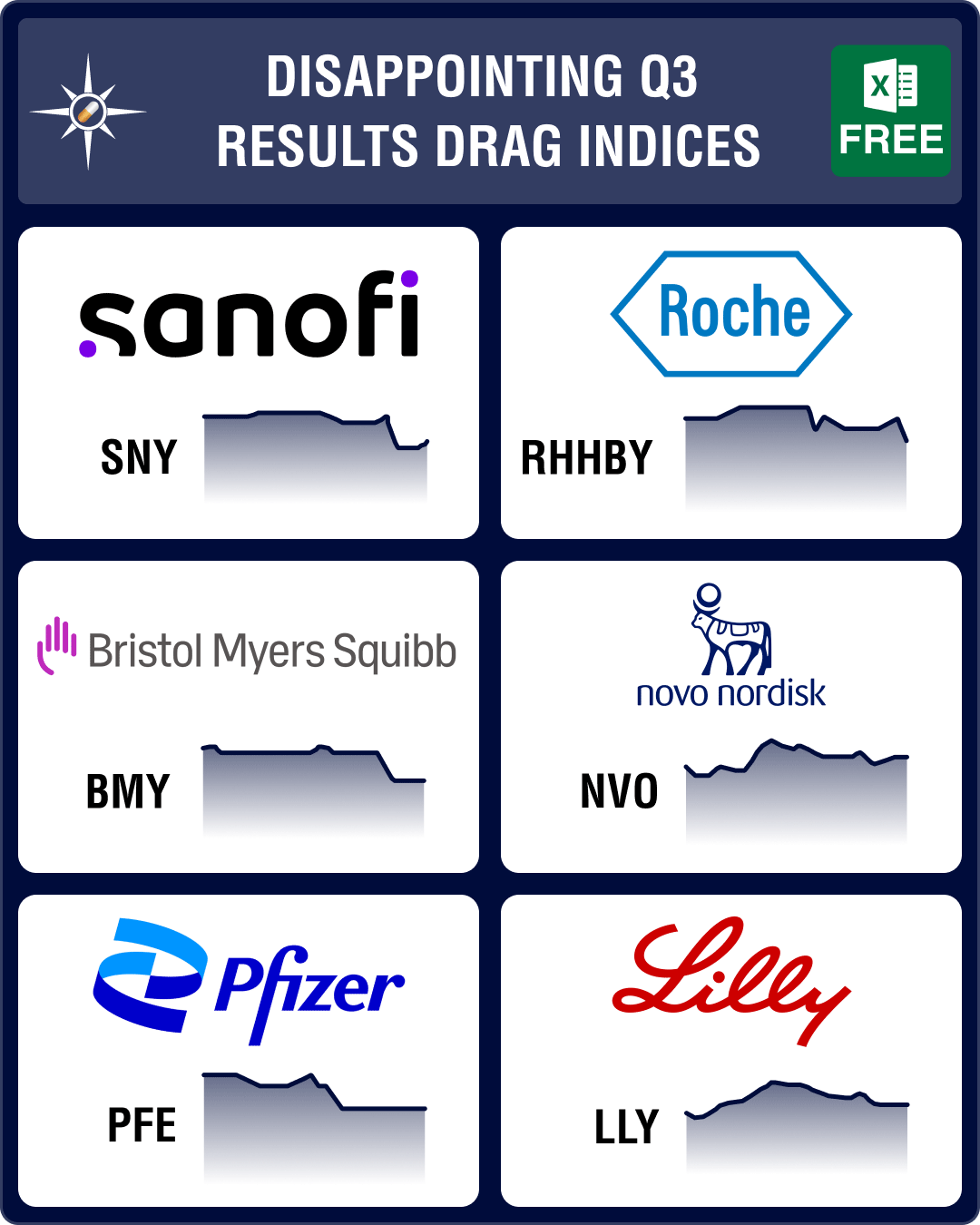
Pipeline Prospector Oct 2023: Disappointing Q3 results, attacks on Israel, Gaza drag pharma indices down further
October was a gloomy month that saw Palestinian militant group Hamas attack Israel in the first week


 Market Place
Market Place Sourcing Support
Sourcing Support