By PharmaCompass
2024-01-11
Impressions: 3022
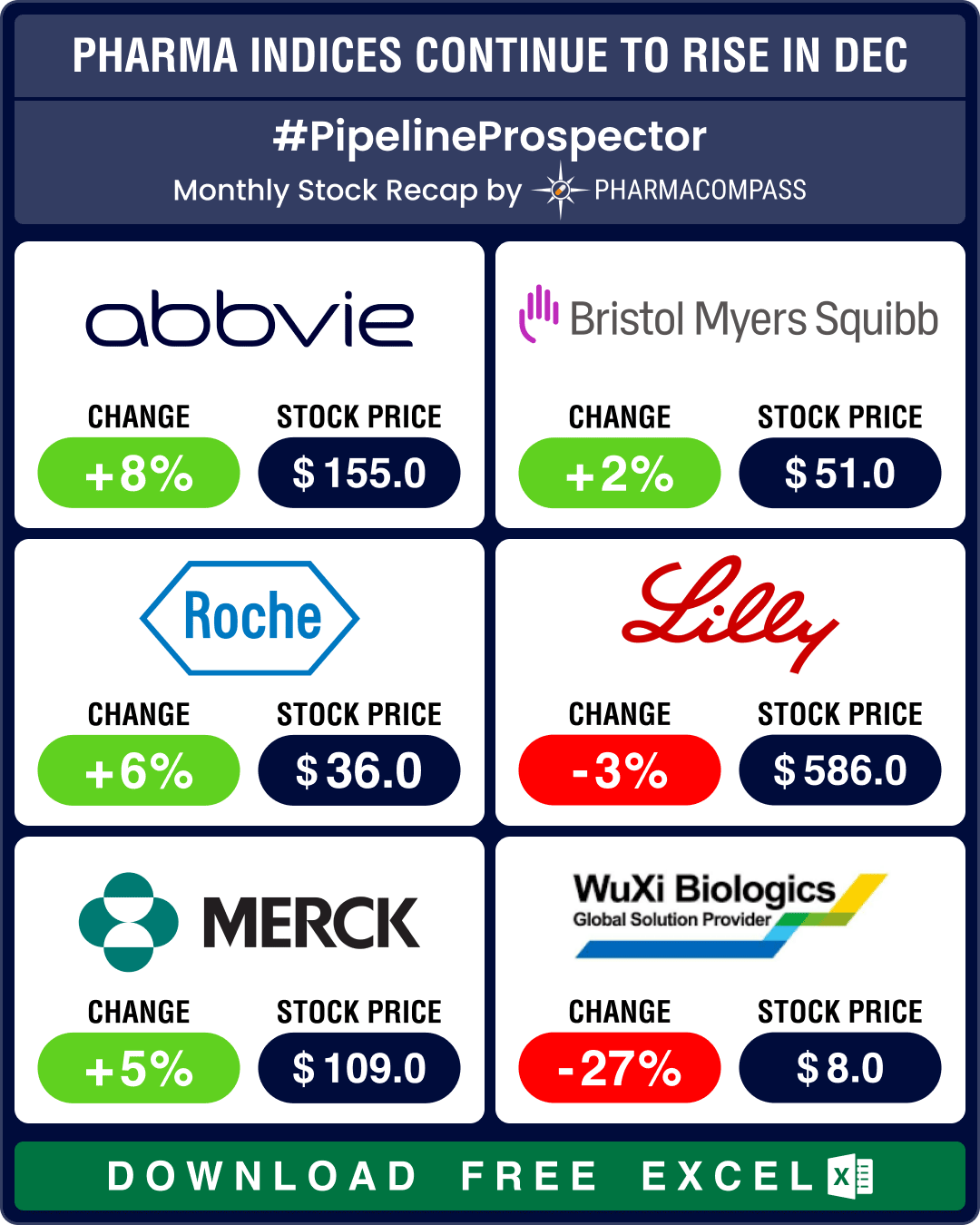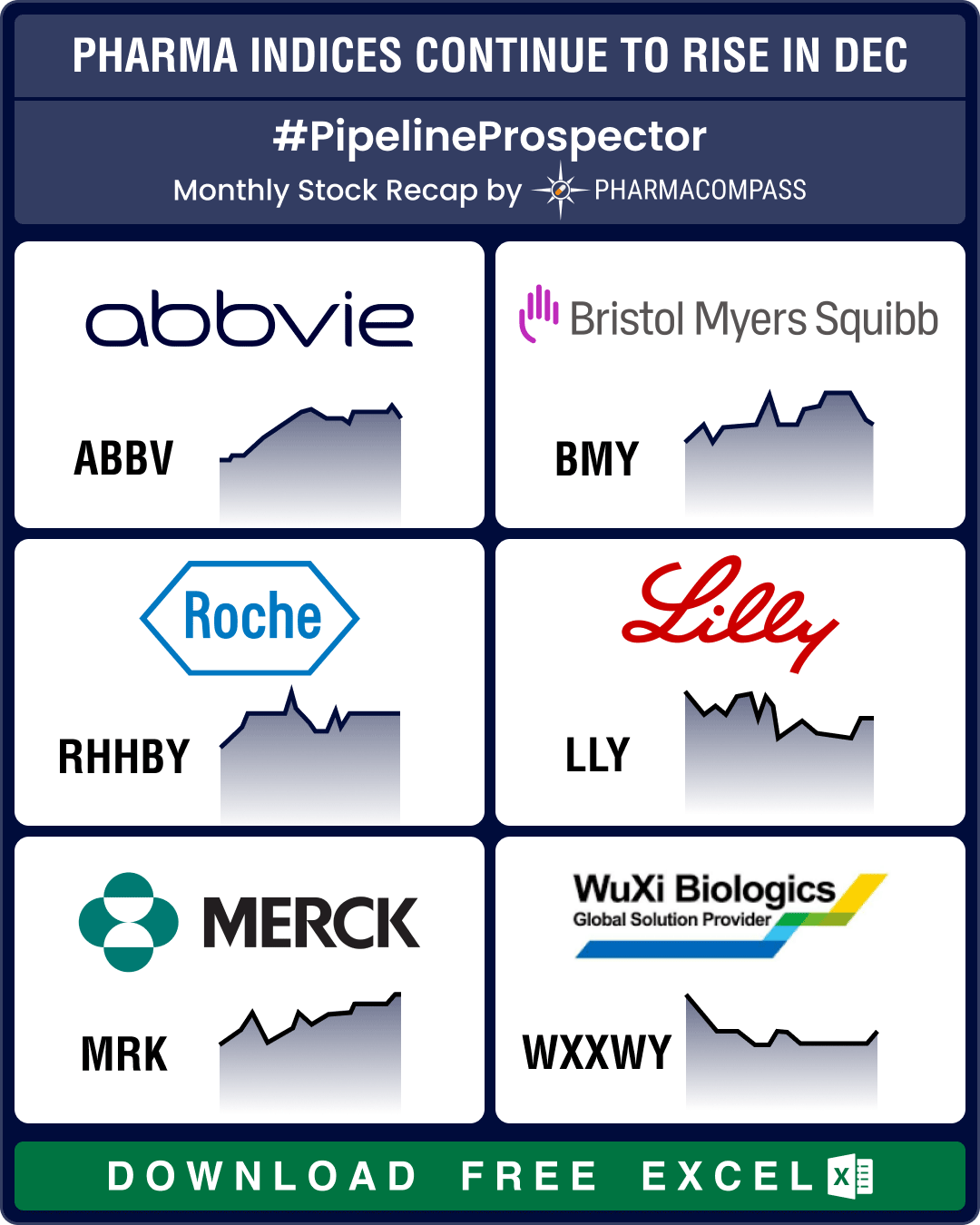
The year 2023 was marked by volatility. The good news is that despite factors like inflation, interest rate hikes and the two ongoing wars, pharma indices ended the year in the green. Though growth was nowhere close to S&P 500’s remarkable 24 percent rise, the Nasdaq Biotechnology Index (NBI) gained a modest 3 percent and rose to 4,370.58, while the SPDR S&P Biotech ETF (XBI) grew 6 percent to 89.29 in 2023. And the S&P Biotechnology Select Industry Index (SPSIBI) ended the year higher by 8 percent, at 6,954.64.
Many saw 2023 as the first year post the pandemic. Clearly, attention turned away from Covid-19 to handling one of the world’s biggest epidemics – obesity. Novo Nordisk and Eli Lilly outshone other drugmakers in 2023, as their newly launched weight management drugs became immensely popular. Falling under the class of drugs known as glucagon-like peptide 1 (GLP-1) agonists, these drugs were originally launched to improve blood sugar control in those with type 2 diabetes.
The year also saw respiratory syncytial virus (RSV) vaccines hitting the markets. Another noteworthy debut was Casgevy, a therapy based on Nobel Prize-winning CRISPR technology that uses molecular “scissors” to trim faulty parts of “sickle” shaped cells that cause the highly debilitating sickle cell disease.
The year’s biggest M&A deal was Pfizer’s acquisition of Seagen for US$ 43 billion. As the year drew to a close, we saw significant M&A activity, with Bristol Myers Squibb acquiring Karuna Therapeutics for US$ 14 billion and RayzeBio for US$ 4.1 billion.
Access the Pipeline Prospector Dashboard for December 2023 Newsmakers (Free Excel)
Lilly, Novo Nordisk leap ahead with anti-obesity drugs; Roche, Astra join GLP-1 race
Eli Lilly saw its sales rise by 17 percent in the first nine months of 2023, at US$ 24.77 billion, on the back of rising sales for Mounjaro (tirzepatide) of US$ 2.96 billion along with other diabetes and weight management drugs during the period.
Novo’s sales rose 33 percent in the same period to DKK 166.4 billion (US$ 24.4 billion) from DKK 128.87 billion (US$ 18.9 billion) in the corresponding period of 2022. Wegovy (semaglutide) accounted for sales of DKK 21.73 billion (US$ 3.19 billion) compared to sales of just DKK 3.74 billion (US$ 0.55 billion) in 2022, a rise of 492 percent. Ozempic’s (semaglutide) sales rose to DKK 65.65 billion (US$ 9.63 billion) in the first nine months of 2023, compared to DKK 42.77 billion (US$ 6.03 billion) in 2022, a rise of 58 percent.
The anti-obesity market is projected to increase to US$ 100 billion by 2030. While existing players are busy expanding capacities for their GLP-1 drugs, more players are eager to enter this market to cash in on the opportunity. In December, Roche bought Carmot Therapeutics for US$ 2.7 billion, thereby getting its hand on CT-388, a promising once-a-week injection currently in mid-stage trials. Similarly, AstraZeneca entered into a potential US$ 2 billion deal with Eccogene for the experimental oral drug ECC5004 for obesity, type-2 diabetes, and other cardio-metabolic conditions.
Merck’s GLP-1 strategy is to come up with treatments whose benefits go beyond weight loss. Its investigational GLP-1 drug efinopegdutide for non-alcoholic steatohepatitis (NASH) demonstrated compelling weight-loss benefits. This experimental drug has won the FDA’s Fast Track designation for treating NASH.
Access the Pipeline Prospector Dashboard for December 2023 Newsmakers (Free Excel)
Pfizer enters ADC space with US$ 43 bn Seagen buy; BMS buys Karuna for US$ 14 bn
After GLP-1 drugs, antibody-drug conjugates (ADCs) have emerged as another big growth area. ADCs are designed as a targeted therapy for treating disease, and are being widely used for the management or treatment of cancer.
Pfizer invested some of the cash it accumulated from its Covid sales to buy out Seagen for US$ 43 billion. The ADC space also saw AbbVie pick up ImmunoGen for US$ 10.1 billion and Merck sign a potential US$ 22 billion deal with Daiichi Sankyo.
The other big deals of 2023 include BMS’ acquisition of Karuna Therapeutics for US$ 14 billion and Merck’s buyout of Prometheus and its potential best-in-class candidate to treat ulcerative colitis and Crohn’s disease for US$ 10.8 billion. Similarly, AbbVie acquired neuroscience drugmaker Cerevel Therapeutics for about US$ 8.7 billion in the hope of shoring up its revenues that have been hit by generic competition to its blockbuster Humira (adalimumab).
Access the Pipeline Prospector Dashboard for December 2023 Newsmakers (Free Excel)
Pfizer, BioNTech, Moderna post lower revenues due to plummeting Covid-19 sales
Pfizer reported a 42 percent drop in revenues in the first nine months of 2023 — at US$ 44.25 billion down from US$ 76.04 billion in the same period of 2022 — owing to disappointing sales of its Covid products. Comirnaty sales dropped 77 percent from US$ 26.48 billion to US$ 5.86 billion, and revenue from Paxlovid was down 73 percent at US$ 4.41 billion, from US$ 17.1 billion in 2022.
Pfizer’s Comirnaty partner BioNTech was worse hit, and saw an 82 percent decline in total sales at € 2.34 billion (US$ 2.56 billion) compared to € 13.03 billion (US$ 14.25 billion) in the first three quarters of 2022. Meanwhile, Moderna’s sales plunged 71 percent in the same period to US$ 4.04 billion, compared to US$ 14.18 billion in 2022.
Access the Pipeline Prospector Dashboard for December 2023 Newsmakers (Free Excel)
FDA okays gene therapies for sickle cell disease, RSV vaccines from GSK, Pfizer
The year saw the United Kingdom and the US approve the world’s first human gene therapy for sickle-cell disease (SCD). The US Food and Drug Administration (FDA) not only approved CRISPR Therapeutics and Vertex Pharmaceuticals’ Casgevy (exagamglogene autotemcel) for SCD, it also approved bluebird bio’s Lyfgenia (lovotibeglogene autotemcel) for the treatment of SCD in patients aged 12 and older who have a history of vaso-occlusive events (when sickled red blood cells block blood flow to the point that tissues become deprived of oxygen).In May, FDA approved GSK’s RSV vaccine Arexvy for people aged 60 and older. Arexvy is the first RSV vaccine to be approved in the US for the common condition that can be fatal for the elderly. Less than a month later, Pfizer’s RSV vaccine Abrysvo also got approved. In July, FDA approved Sanofi and AstraZeneca’s preventive RSV antibody therapy Beyfortus (nirsevimab-alip) for newborns and infants. RSV is the number one cause of infant hospitalization in the US.
Access the Pipeline Prospector Dashboard for December 2023 Newsmakers (Free Excel)
Our view
A strong portfolio and drug pipeline can help drugmakers sail through the toughest economic and regulatory environment. While it’s difficult to say how things will pan out in 2024, we do know that several pathbreaking drugs are coming up for approval by the FDA early this year, including Madrigal Pharmaceuticals’ resmetirom (the first treatment for NASH with liver fibrosis), Merck’s sotatercept (a treatment for pulmonary arterial hypertension) and, later, in September, Karuna Therapeutics’ drug to treat schizophrenia. In short, there is much to look forward to in 2024.
Access the Pipeline Prospector Dashboard for December 2023 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in December 2023
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Pharma & Biotech Newsmakers in December 2023 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







