By PharmaCompass
2025-01-09
Impressions: 11182
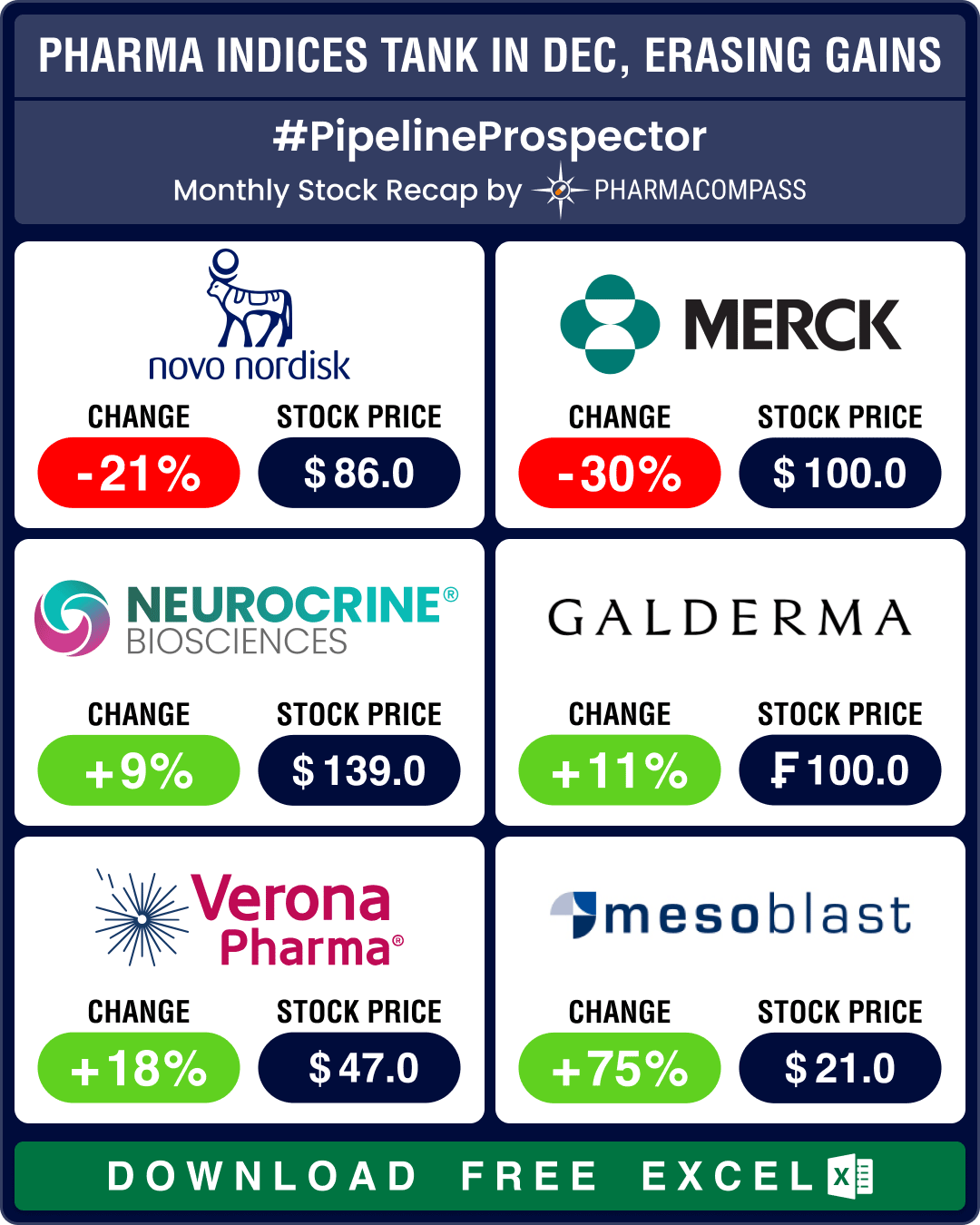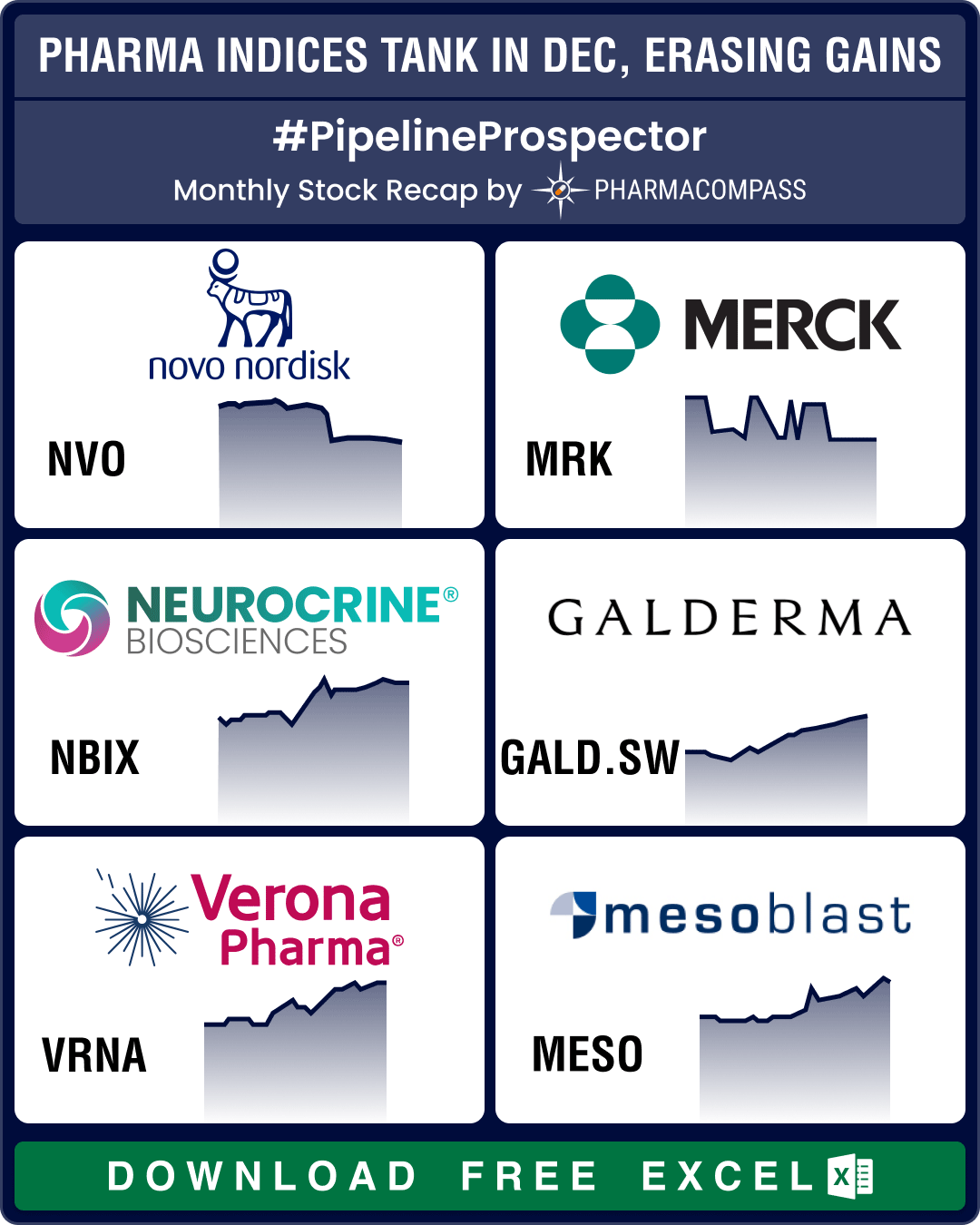
December proved to be one of the most bearish months of 2024 for the biopharma sector. The Nasdaq Biotechnology Index (NBI) sank 7.2 percent from 4,638.6 to 4,310.6. The SPDR S&P Biotech ETF (XBI) plummeted 9.6 percent (from 99.29 to 90.06) and the S&P Biotechnology Select Industry Index (SPSIBI) plunged 9.5 percent (from 7,763.7 to 7,023).
This downturn in indices at the fag-end of 2024 effectively erased the gains they had made during the year. NBI was lower by 0.68 percent at the end of 2024, as opposed to 2023. XBI and SPSIBI posted modest gains of 1.84 percent and approximately 1 percent, respectively. The sector’s tepid performance stands in stark contrast to the broader market, which closed 2024 at near-record highs.
Access the Pipeline Prospector Dashboard for December 2024 Newsmakers (Free Excel)
GLP-1 drugs show promise beyond obesity, diabetes; Novo Holdings completes US$ 16.5 bn Catalent buyout
Glucagon-like peptide-1 (GLP-1) agonists created much news in 2024, for not just their demand and concomitant shortages, but also for their health benefits beyond weight-loss and diabetes.
December saw Lilly’s Zepbound break new ground as the first FDA-approved treatment for obstructive sleep apnea (OSA) in adults with obesity. OSA affects around 1 billion people globally.
In March 2023, Novo’s Wegovy became the first obesity drug cleared by the FDA to lower the risk of cardiovascular death, heart attack, and stroke. In the same month, Novo’s blockbuster Ozempic also slashed the risk of kidney disease progression in a late-stage trial.
The biggest investments in December came from Novo Nordisk and Eli Lilly — the two early entrants into the potential US$ 150 billion obesity market. Throughout 2024, the two companies kept injecting billions to boost production in order to meet the burgeoning demand. Just last month, Lilly invested US$ 3 billion to expand its recently acquired injectables plant in Wisconsin, US, to meet the soaring demand for GLP-1 drugs. And Novo invested US$ 1.2 billion to establish a new rare disease drugs plant in Odense, Denmark.
After much ado, Novo Holdings completed its US$ 16.5 billion acquisition of Catalent in December, having won the greenlight from both the US Federal Trade Commission and European Commission. This was the biggest pharma deal of 2024 (announced in February).
Interestingly, Lilly turned out to be the best performing pharma stock of the year (its market cap increased by a whopping US$ 163 billion in 2024) while Novo’s stock ended the year 17 percent lower than 2023. It crashed 21 percent in December after disappointing results from a late stage trial of its eagerly awaited experimental next-generation obesity drug CagriSema.
Access the Pipeline Prospector Dashboard for December 2024 Newsmakers (Free Excel)
Rise of ‘buyout-hesitant’ biotechs that bagged maiden approvals; Novartis snaps up PTC’s Huntington’s disease program
Novartis has been investing in early-stage science. The Swiss drugmaker inked over 20 deals in 2024, paying over US$ 5.5 billion upfront and promising over US$ 25 billion in biobucks. In December, Novartis snapped up PTC’s Huntington’s disease program in a US$ 2.9 billion deal, including US$ 1 billion upfront.
Barring the Novo-Catalent buyout, all other deals in the biopharma space last year were in the sub-US$ 5 billion range. The other major deals were Vertex’s US$ 4.9 billion acquisition of Alpine Immune, Gilead’s US$ 4.3 billion buyout of CymaBay and Lilly’s US$ 3.2 billion acquisition of Morphic Holding.
The most striking trend was the emergence of a new breed of ‘buyout hesitant' biotech firms that preferred to commercialize their products independently instead of seeking Big Pharma partnerships. This shift in strategy proved successful for many, as numerous companies completed the transition from clinical-stage to commercial-stage operations.
December saw several such notable commercial debuts. Ionis Pharmaceuticals secured approval of Tryngolza, the first-ever treatment for familial chylomicronemia syndrome (FCS), a rare genetic disorder. Mesoblast’s cell therapy Ryoncil gained US approval for treating post-transplant complications (graft-versus-host disease), offering a novel approach to managing immune-related adverse events. Mesoblast’s stock grew 950 percent in 2024.
Similarly, Checkpoint Therapeutics (stock up 50 percent in 2024) secured FDA approval for Unloxcyt, targeting cutaneous squamous cell carcinoma in patients with locally advanced or metastatic disease where surgery and radiation are not viable options.
Merus’ Bizengri brought new options to patients with hard-to-treat cancers, adding to the growing arsenal of precision oncology treatments. Merus’ stock ballooned 48 percent last year.
Though the trend caught momentum in December, even the preceding months of 2024 saw significant debuts. For instance, in March, Madrigal’s Rezdiffra became the first drug approved in the US for the common fatty liver disease known as metabolic dysfunction-associated steatohepatitis (MASH). Madrigal’s stock grew 35 percent last year.
Similarly, Verona brought to market Ohtuvayre (approved in June), the first new mechanism of action in over two decades for the treatment of chronic obstructive pulmonary disease. Verona’s stock soared 147 percent in 2024. And in May, PTC’s Kebilidi, a treatment for enzyme deficiency disorder, became the first FDA-approved gene therapy directly administered to the brain. PTC’s stock rose 68 percent in 2024.
Access the Pipeline Prospector Dashboard for December 2024 Newsmakers (Free Excel)
Drug approvals gain momentum in December; Vertex, Neurocrine, Astra score wins
The year 2024 saw some landmark drug approvals. And as the year drew to a close, FDA began approving drugs at a feverish pace. Novo won approval for Alhemo, a once-daily subcutaneous injection that significantly reduces bleeding episodes in hemophilia patients, marking a shift from more burdensome treatment regimens.
Vertex Pharmaceuticals expanded treatment options for cystic fibrosis patients with its triple combination therapy that targets specific genetic variants of the disease.
Dermatology saw two advancements with the approvals of Organon’s Vtama cream and Galderma’s Nemluvio injection, both targeting atopic dermatitis through distinct mechanisms. FDA also approved Neurocrine Biosciences’ Crenessity — the first new treatment in 70 years for classic congenital adrenal hyperplasia, a rare hormonal disorder.
The oncology landscape saw Bristol Myers-Squibb enhance patient convenience with Opdivo Qvantig, an injectable formulation of its blockbuster cancer drug. And FDA expanded the approval of AstraZeneca’s Imfinzi to treat adults with limited-stage small cell lung cancer.
Access the Pipeline Prospector Dashboard for December 2024 Newsmakers (Free Excel)
Our view
The industry dynamics witnessed a marked shift in 2024. The absence of mega-mergers, coupled with Novartis’ approach to buy smaller companies, suggests a more measured approach to deal-making. The emergence of companies like Madrigal, Verona, and Ionis signals a maturing biotech sector, less dependent on Big Pharma partnerships. Looking ahead, this evolution could herald a more diverse and resilient industry landscape.
Access the Pipeline Prospector Dashboard for December 2024 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in December 2024
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : PHARMA INDICES TANK IN DEC, ERASING GAINS by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







