By PharmaCompass
2024-05-09
Impressions: 2086
Pharma indices had begun to recede in March. Their red streak accelerated in April with the Nasdaq Biotechnology Index (NBI) falling 6 percent from 4429.97 in March to 4162.33 last month and the SPDR S&P Biotech ETF (XBI) index tripping 10 percent from 94.34 to 84.62. Similarly, the S&P Biotechnology Select Industry Index (SPSIBI) was down 11 percent from 7402.50 to 6584.40 in April.
This muted performance was also reflected in the first quarter (Q1) results. Biogen, Biomarin, Roche, Gilead, Merck, Sanofi, BMS and Johnson & Johnson announced their Q1 results in April. While Roche and Biogen reported a drop in revenues, BMS posted a loss in Q1, even though its revenues increased. And Sanofi reported a 14.7 percent drop in operating income due to competition from generics and currency fluctuations. BioMarin, Gilead and Merck reported a 9 percent, 5 percent and 9 percent increase, respectively, in their Q1 revenues over Q1 2023.
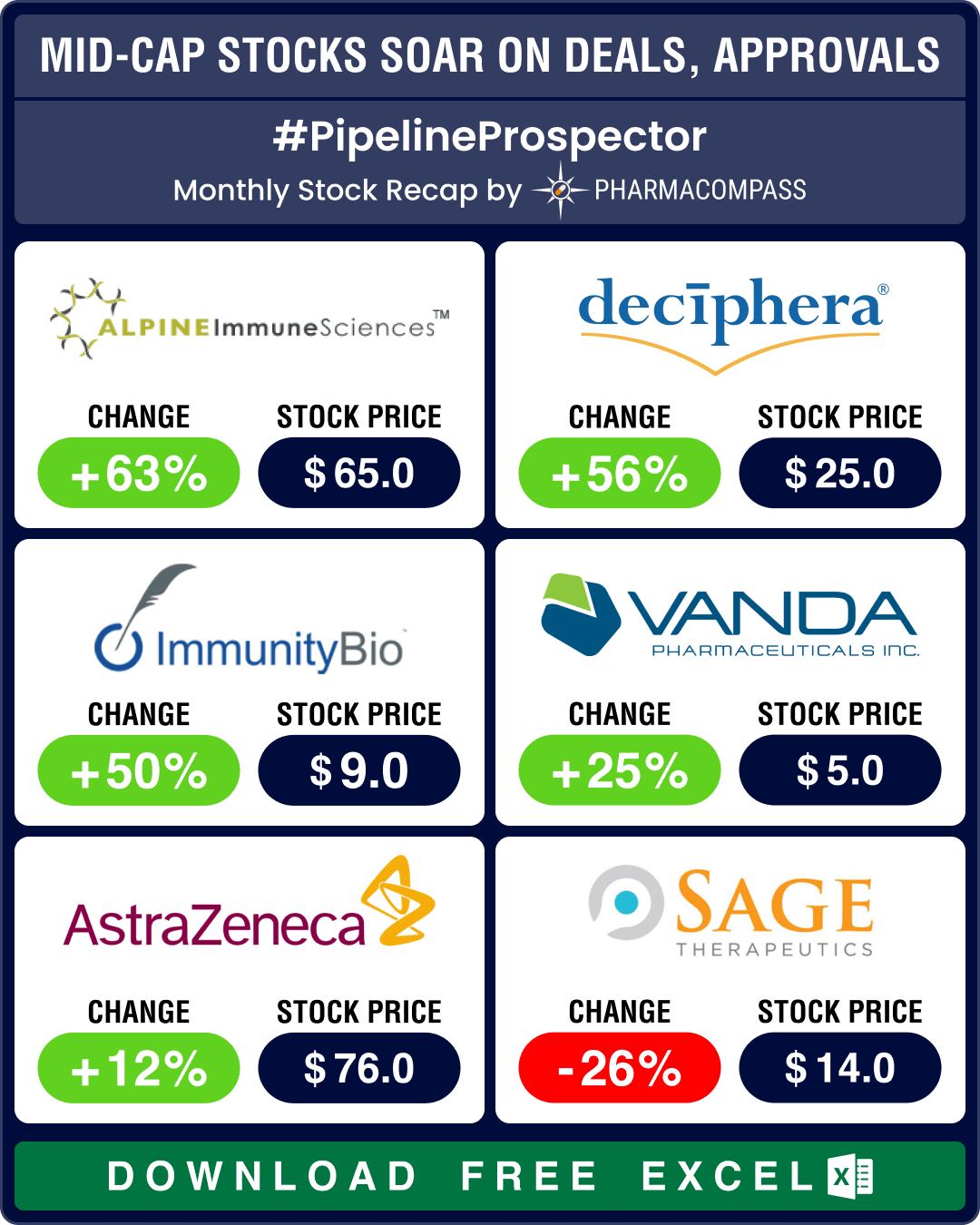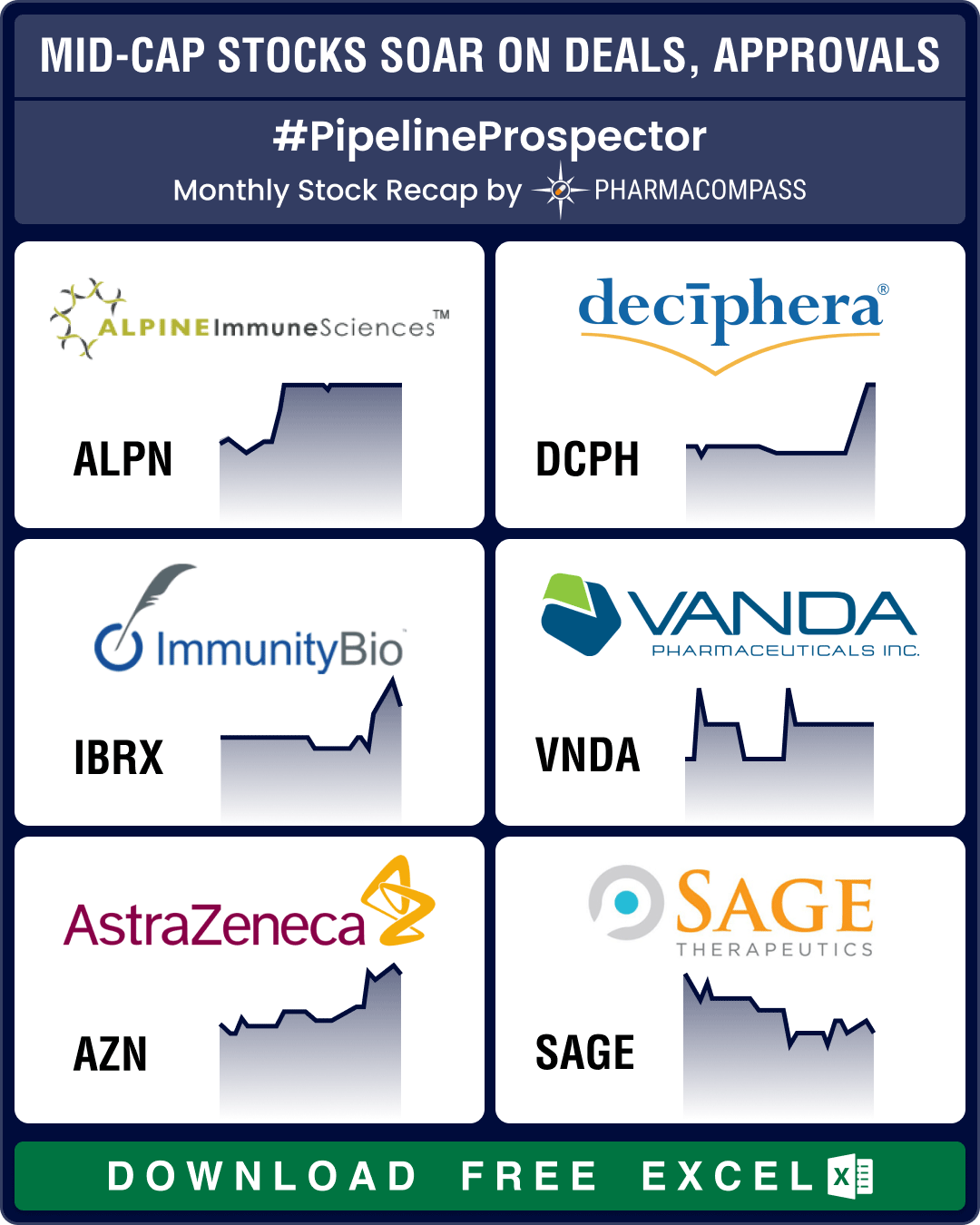
There were a handful of deals announced last month, the biggest being Vertex Pharmaceuticals’ acquisition of Alpine Immune Sciences for US$ 4.9 billion. The deal gives Vertex access to Alpine’s protein-based immunotherapies for autoimmune diseases.
Japanese drugmaker Ono Pharmaceutical agreed to acquire Massachusetts-based Deciphera Pharmaceuticals for US$ 2.4 billion. The acquisition gives Ono access to Deciphera's oncology pipeline. Denmark’s Genmab agreed to acquire privately-owned ProfoundBio for US$ 1.8 billion. ProfoundBio has three next-generation antibody drug conjugate (ADC) candidates in its pipeline that will now go to Genmab.
Access the Pipeline Prospector Dashboard for April 2024 Newsmakers (Free Excel)
FDA okays ImmunityBio’s Anktiva, Day One’s Ojemda for bladder, brain cancer
Like March, April too saw several significant drug approvals, especially in oncology. ImmunityBio (its stock was up 50 percent in April) won its first US Food and Drug Administration (FDA) approval as the agency greenlit Anktiva as part of a combination therapy to treat a type of bladder cancer. With this approval, Anktiva will compete with Merck’s Keytruda.
AstraZeneca (stock up 12 percent) and Daiichi Sankyo’s Enhertu has been granted accelerated approval in the US to treat adult patients with HER2-positive solid tumors that have spread or cannot be surgically removed. These patients have undergone prior treatment and have no satisfactory alternatives available to them.
J&J and Legend’s Carvykti has become the first and only BCMA-targeted therapy approved by the FDA for patients with relapsed or refractory multiple myeloma, who have received just one prior line of treatment. The approval is an important milestone in J&J’s plans to make Carvykti a US$ 5 billion-plus asset at peak yearly sales.
Day One Biopharmaceuticals’ Ojemda has been granted FDA’s accelerated approval to treat certain types of pediatric brain cancer. The pan-RAF kinase inhibitor has been okayed for patients six months of age and older with relapsed or refractory pediatric low-grade glioma (LGG) harboring a BRAF fusion or rearrangement, or BRAF V600 mutation. This is the first FDA approval of a systemic therapy for treating what is the most common form of childhood brain tumor.
Access the Pipeline Prospector Dashboard for April 2024 Newsmakers (Free Excel)
Basilea’s antibiotic bags FDA nod; Vanda’s Fanapt approved for bipolar disorder
The agency also approved Vanda Pharmaceuticals’ (stock up 25 percent) Fanapt tablets for treating manic or mixed episodes associated with bipolar-1 disorder in adults. The atypical antipsychotic agent has been used for the treatment of patients with schizophrenia since its FDA approval in 2009. This second approval for bipolar-1 disorder patients (who experience manic episodes that last at least seven days) could help revive Fanapt prescriptions.
FDA has also approved Basilea Pharmaceutica’s Zevtera, an antibiotic for bacterial infections including multidrug-resistant strains. The US agency has okayed it for three conditions – treatment of adults with Staphylococcus aureus bacteremia, including those with right-sided infective endocarditis; adults with acute bacterial skin and skin structure infections; and adult and pediatric patients with community-acquired bacterial pneumonia.
Access the Pipeline Prospector Dashboard for April 2024 Newsmakers (Free Excel)
BMS’ schizo drug shows benefit sans weight gain; Imfinzi scores in lung cancer trial
A common side effect associated with anti-psychotics is weight gain. However, interim results from a late-stage trial have shown that BMS’ schizophrenia drug KarXT continued to improve symptoms of the severe mental disease even at 52 weeks, and without weight gain.
In another phase 3 trial, Imfinzi significantly improved overall survival and progression-free survival in patients with limited-stage small cell lung cancer. This makes this blockbuster drug the first and only immunotherapy to demonstrate survival benefit for this aggressive form of lung cancer. Imfinzi had posted sales of US$ 4.24 billion in 2023.
Intra-Cellular Therapies said a phase 3 study of its drug Caplyta in people with major depressive disorder (MDD) met both its primary and key secondary endpoints. Given once daily as an adjunctive therapy to antidepressants, Caplyta 42 mg significantly beat the placebo at improving depression severity.
Access the Pipeline Prospector Dashboard for April 2024 Newsmakers (Free Excel)
Zepbound shows benefit in sleep disorder; Novartis’ Fabhalta reduces proteinuria
Eli Lilly’s popular weight-loss drug Zepbound cut irregular breathing episodes associated with a common sleep-related disorder – obstructive sleep apnea (OSA). Treatment with Zepbound reduced the frequency of irregular breathing episodes by as much as 63 percent in adults with OSA and obesity across two late-stage trials. Lilly plans to submit the findings to FDA to expand Zepbound’s use for OSA.
Roche’s Genentech said its drug Columvi, in combination with chemotherapy, demonstrated a statistically significant improvement in overall survival for people with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). A late-stage study pitted the Columvi-chemotherapy combination against a rituximab-chemotherapy combination in patients who received at least one prior line of therapy and are not candidates for autologous stem cell transplant. Patients in the Columvi group lived longer.
In another late-stage trial, Novartis' drug Fabhalta reduced proteinuria (protein in the urine) in patients with IgA nephropathy by 38.3 percent. The Swiss drugmaker filed for FDA’s accelerated approval using the data, and the agency has granted it priority review.
Access the Pipeline Prospector Dashboard for April 2024 Newsmakers (Free Excel)
Our view
At the start of 2024, the US market saw inflation falling and there were talks of interest rate cuts by the Federal Reserve. But few months on, the outlook has darkened a bit with inflation refusing to let off steam and economic output slowing down in the US. This is getting reflected in the pharma indices and the Q1 results of several drugmakers. Though overall, we have faith in the pharma sector’s spirit of innovation and its zest for dealmaking. Despite the macroeconomic challenges, there is hope that the other three quarters of 2024 will look better.
Access the Pipeline Prospector Dashboard for April 2024 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in Apr 2024
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Pharma & Biotech Newsmakers in Apr 2024 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







