By PharmaCompass
2024-09-05
Impressions: 2196
As summer draws to a close, pharma and biotech indices posted their fourth consecutive month in the green. The Nasdaq Biotechnology Index (NBI) rose 1.3 percent from 4,821.49 to 4,882 in August and the SPDR S&P Biotech ETF (XBI) index gained 1.7 percent from 99.53 to 101.26. The S&P Biotechnology Select Industry Index (SPSIBI) saw a 2.3 percent increase to 7,897.85 from 7,717 posted in July-end. Over the last four months, NBI, XBI, and SPSIBI have rallied 17 percent, 19 percent, and 20 percent, respectively.
Amongst the notable negative news, the US Food and Drug Administration (FDA) declined to approve an application of MDMA, commonly known as ecstasy, to treat post-traumatic stress disorder (PTSD). Lykos Therapeutics, the company behind this application, received a complete response letter citing concerns about the trial.
Access the Pipeline Prospector Dashboard for August 2024 Newsmakers (Free Excel)
Otsuka buys Jnana for up to US$ 1.1 bn; Merck inks US$ 1.3 bn deal with China’s Curon
August saw several acquisitions and deals. Japan’s Otsuka Pharmaceutical said it is acquiring clinical-stage biotech Jnana Therapeutics through a potential US$ 1.1 billion deal. This includes a payment of US$ 800 million to Jnana’s shareholders on completion of the acquisition, and an additional US$ 325 million in milestone payments.
Merck has evinced interest in the growing field of bispecific antibodies by paying China-based Curon US$ 700 million upfront, with an additional US$ 600 million in milestone payments, for the rights to CN201, an experimental cancer med in early-stage trials for treating non-Hodgkin’s lymphoma and B-cell acute lymphocytic leukemia.
Roche, via its subsidiary Genentech, has secured exclusive rights to molecules from Sangamo Therapeutics designed to repress the gene that makes “tau,” a protein many scientists believe is a driver of Alzheimer’s disease. The potential US$ 1.95 billion deal comprises other novel genomic medicines for neurodegenerative diseases.
Denmark’s Adcendo acquired global rights (excluding Greater China) to Multitude Therapeutics' first-in-class antibody-drug-conjugate (ADC) candidate for up to US$ 1 billion. The ADC targets tissue factor (TF) highly expressed in various cancers including lung, colorectal, cervical, esophageal, head and neck, bladder, and some gastrointestinal cancers, but limited in normal tissues. The candidate, ADCE-T02, is a highly differentiated anti-TF ADC.
In other deals, Instil Bio agreed to pay up to US$ 2 billion to China’s ImmuneOnco Biopharmaceuticals for two clinical-stage cancer candidates. Similarly, Eisai inked a deal with SEED Therapeutics worth up to US$ 1.5 billion to develop novel drugs for neurodegenerative diseases and cancer.
Access the Pipeline Prospector Dashboard for August 2024 Newsmakers (Free Excel)
Gilead’s US$ 4.3bn CymaBay bet pays off; Adaptimmune’s Tecelra becomes first-ever TCR gene therapy
The month also saw several significant drug approvals. Gilead’s Livdelzi gained FDA’s accelerated approval for primary biliary cholangitis (PBC), an inflammatory liver disease. This approval validates Gilead’s US$ 4.3 billion acquisition of CymaBay and positions the once-daily pill as a potential challenger to the current PBC standard of care.
Novartis’ Fabhalta also gained accelerated approval for reducing excess protein in the urine of patients with primary immunoglobulin A nephropathy (IgAN), addressing an important aspect of kidney disease management.
Adaptimmune’s Tecelra received accelerated approval from the FDA as the first-ever T cell receptor (TCR) gene therapy. It was greenlit for a rare type of cancer — synovial sarcoma — that often affects young people.
J&J’s high hopes for Rybrevant got validated when FDA approved it for use in combination with its new drug Lazcluze to treat a kind of non-small cell lung cancer (NSCLC). This is the only chemotherapy-free regimen that has shown superior progression-free survival as compared to AstraZeneca’s Tagrisso, the current standard of care in the first-line setting.
Meanwhile, Astra’s other blockbuster cancer drug Imfinzi received FDA’s approval as an additional treatment after surgery for a type of NSCLC, expanding its use in the treatment paradigm.
Servier’s Voranigo became the first and only treatment in the US for a certain kind of brain tumor, offering a once-daily pill option for patients with grade 2 IDH-mutant glioma. Additionally in oncology, GSK’s Jemperli received a broad US label expansion for first-line treatment of endometrial cancer. Citius’ Lymphir received FDA approval for relapsed or refractory cutaneous T-cell lymphoma.
ARS Pharmaceuticals’ EURneffy and Neffy became the first nasal spray alternatives to EpiPen for severe allergic reactions in Europe and the US, respectively. This represents a new era in needle-free emergency allergy treatment.
To deal with a surge in Covid cases in the US, FDA approved updated versions of Pfizer and BioNTech’s Comirnaty, Moderna’s Spikevax and Novavax's jab that target a strain called KP.2.that target a strain called KP.2.
Access the Pipeline Prospector Dashboard for August 2024 Newsmakers (Free Excel)
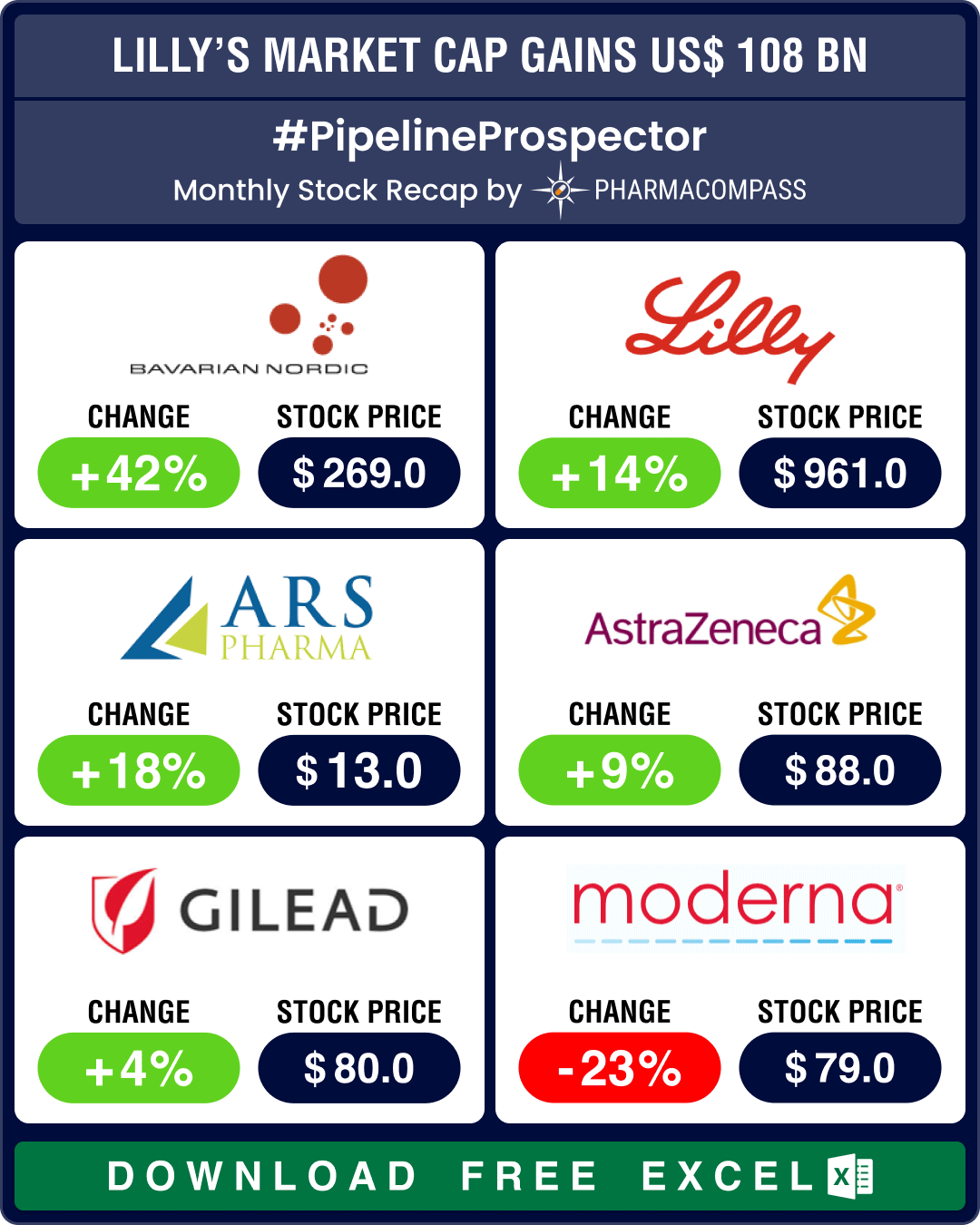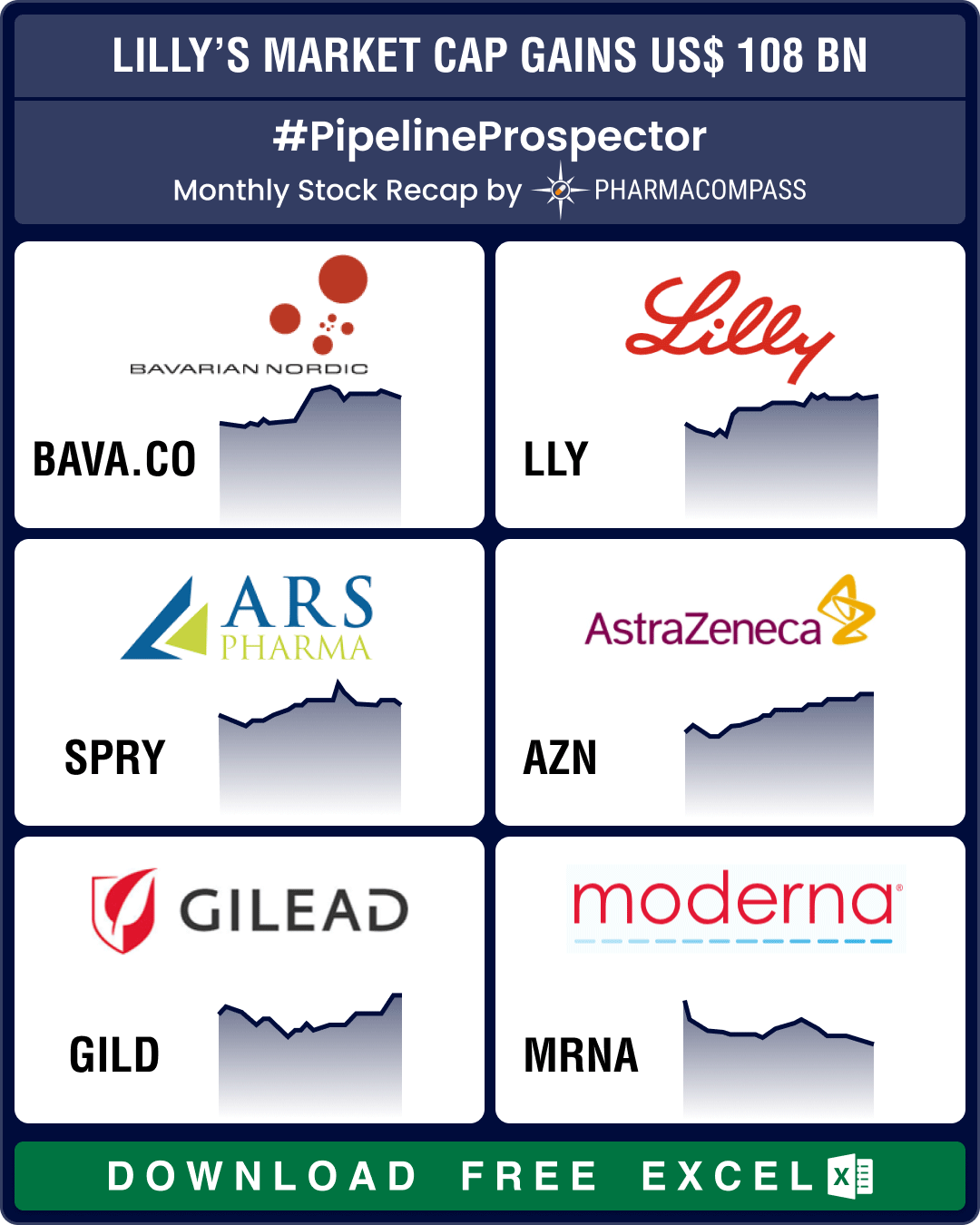
Lilly’s market cap surges US$ 108 bn post Q2 results; Bavarian Nordic’s stock jumps 42%
Eli Lilly announced its second-quarter results last month. Its Q2 revenue increased 36 percent year-on-year due to its diabetes and obesity meds Mounjaro and Zepbound and breast cancer med Verzenio. It prompted Lilly to raise its 2024 revenue guidance by US$ 3 billion. Lilly now expects between US$ 45.4 billion and US$ 46.6 billion in 2024 revenue. The news led to a 14 percent rise in its stock, as it gained over US$ 108 billion in market capitalization. The stock hit an all-time high of US$ 972.53 on August 22.
Lilly said tirzepatide (Zepbound and Mounjaro) slashed the risk of developing type 2 diabetes in overweight or obese adults with pre-diabetes by 94 percent. One in three adults in the US, or around 98 million Americans, have pre-diabetes. A late-stage trial also showed tirzepatide reduced the risk of hospitalization or death due to heart failure by 38 percent.
The stock of Bavarian Nordic, which makes the monkey pox vaccine Jynneos, gained 42 percent in August after the World Health Organization declared a global health emergency over the mpox outbreak in Africa. As the month drew to a close, FDA granted expanded approval to Emergent BioSolutions’ smallpox vaccine — ACAM2000 — for use in people at high risk of mpox infection. This makes Emergent’s shot the second approved vaccine against mpox in the US after Jynneos.
In trials, Bayer posted a key win with Kerendia showing it can reduce the risk of cardiovascular death, and first and recurrent heart failure events in a phase 3 trial.
Access the Pipeline Prospector Dashboard for August 2024 Newsmakers (Free Excel)
Our view
During August, the pharmaceutical industry’s resilience was on full display. A surge in Lilly’s market cap by US$ 108 billion underscores the commercial potential of cutting-edge therapies. And we hope to see more of such successes on the bourses in the coming months.
Access the Pipeline Prospector Dashboard for August 2024 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in August 2024
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : LILLY’S MARKET CAP GAINS US$ 108 BN by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







