By PharmaCompass
2025-02-06
Impressions: 3739
January was a busy month that saw several deals being announced at the JP Morgan Healthcare Conference. In all, deals worth over US$ 19.3 billion were signed at the annual event. The month also saw several key drugs getting approved by the US Food and Drug Administration (FDA).
This got reflected in the indices — while the Nasdaq Biotechnology Index (NBI) rebounded 4.42 percent to reach 4,532.75, the SPDR S&P Biotech ETF (XBI) and S&P Biotechnology Select Industry Index (SPSIBI) posted gains of 1.89 percent and 2.84 percent respectively.
Access the Pipeline Prospector Dashboard for January 2025 Newsmakers (Free Excel)
J&J buys Intra-Cellular for US$ 14.6 bn, Lilly strikes US$ 2.5 bn cancer deal with Scorpion
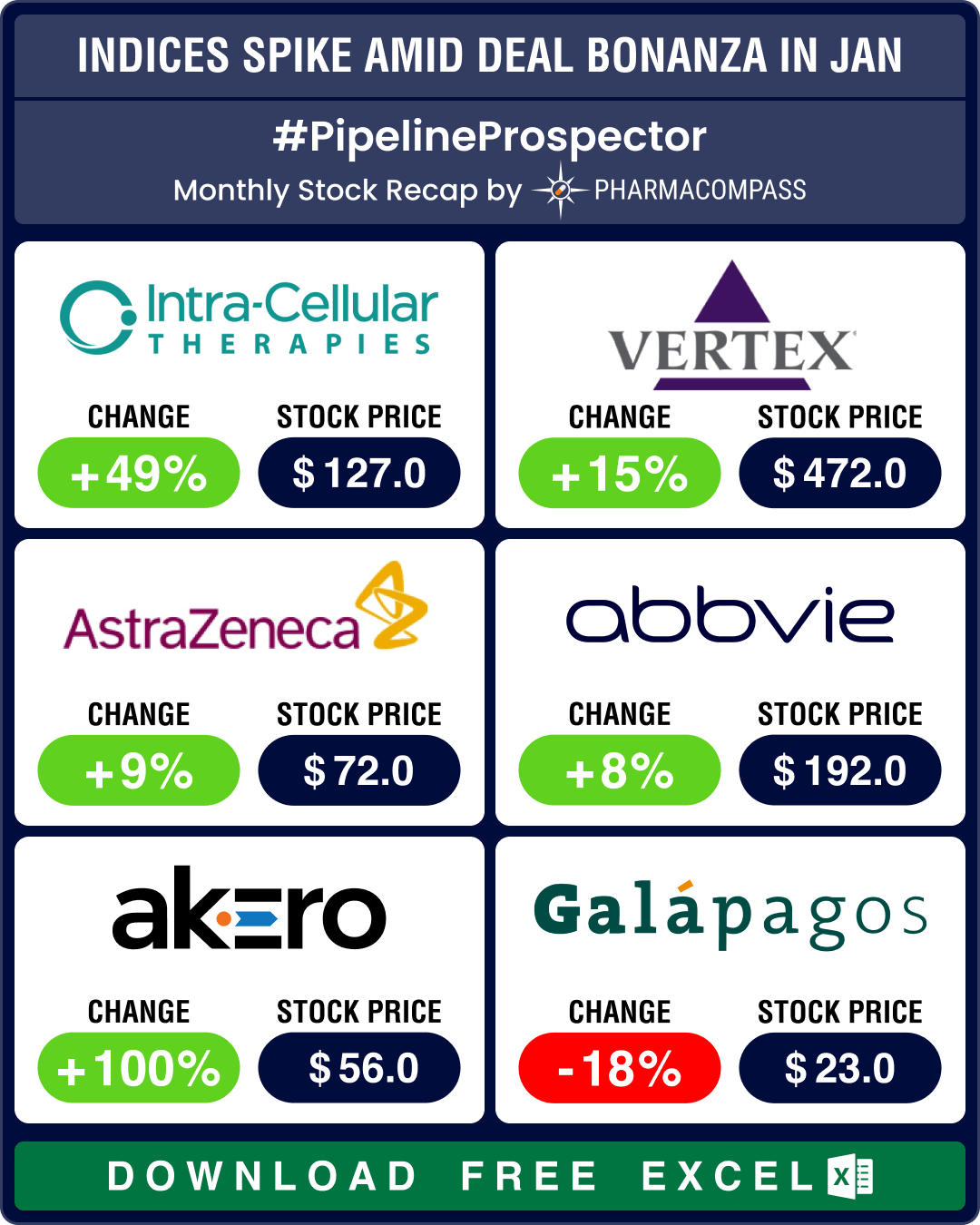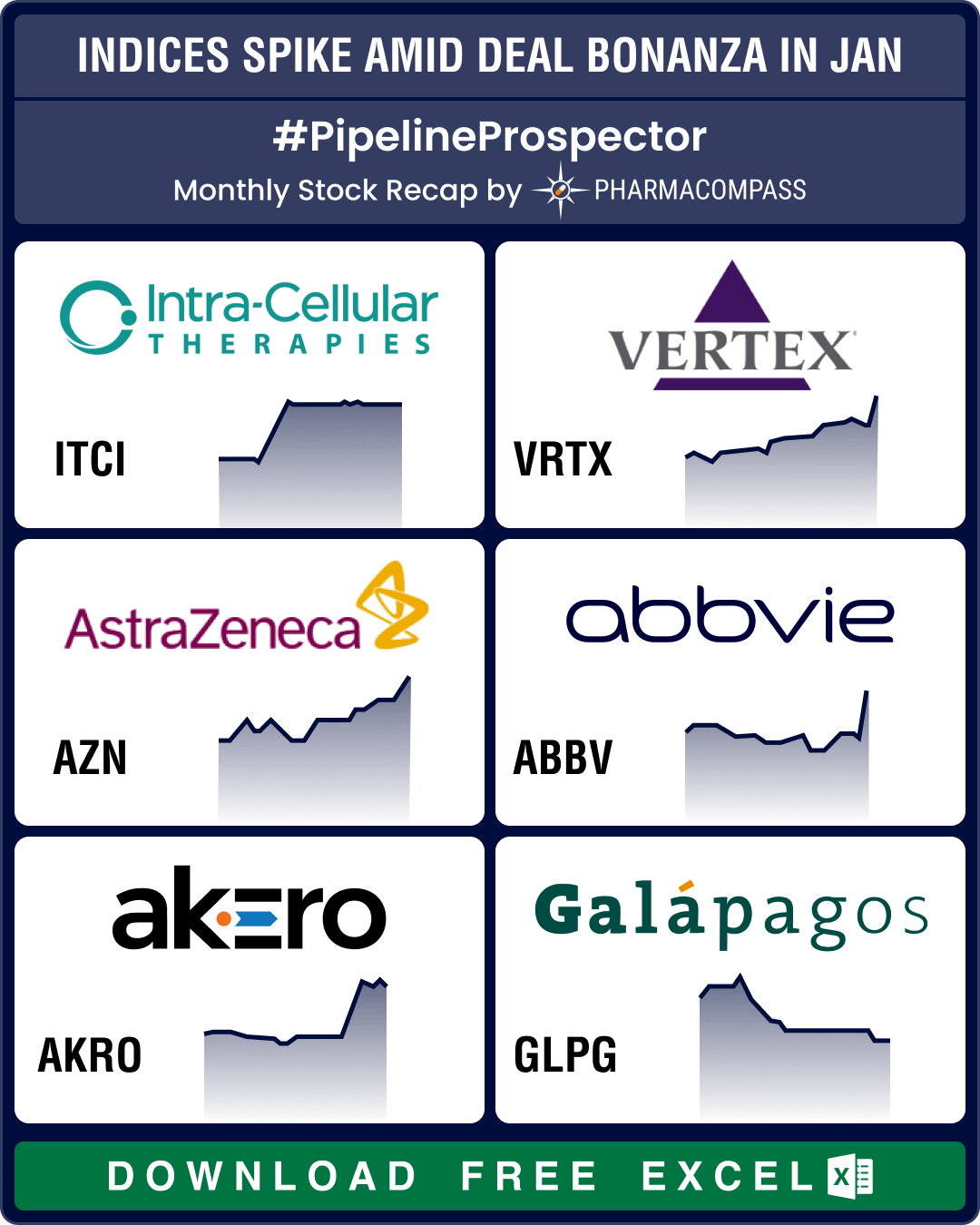
Johnson & Johnson (stock up 5 percent) announced the biggest biotech buyout since March 2023 with its acquisition of Intra-Cellular Therapies (stock up 49 percent), a neuroscience-focused biopharmaceutical company, for US$ 14.6 billion. Intra-Cellular’s lead drug, Caplyta (lumateperone), approved for bipolar depression, is undergoing FDA review for major depressive disorder (MDD).
Eli Lilly (stock up 5 percent) announced a US$ 2.5 billion deal to acquire Scorpion Therapeutics’ experimental cancer therapy, STX-478. This therapy targets specific mutations in breast cancer and other solid tumors. Lilly also partnered with UK-based Alchemab Therapeutics to develop up to five new antibodies targeting amyotrophic lateral sclerosis (ALS).
Gilead Sciences (stock up 5 percent) and LEO Pharma teamed up in a deal worth up to US$ 1.7 billion to develop oral STAT6 inhibitors for inflammatory diseases. These inhibitors will target key pathways in conditions like atopic dermatitis, asthma, and COPD, offering a potential oral alternative to injectable biologics.
GSK (stock up 6 percent) agreed to acquire IDRx, a Massachusetts-based developer of rare cancer therapies, for up to US$ 1.15 billion. IDRx focuses on treatments for gastrointestinal stromal tumors (GIST), rare cancers that develop in the digestive tract.
Access the Pipeline Prospector Dashboard for January 2025 Newsmakers (Free Excel)
Ozempic becomes first GLP-1 to treat CKD in diabetes patients; Novo puts another US$ 4.6 bn in deal with Valo
FDA approved Novo Nordisk’s Ozempic (semaglutide) to reduce the risk of kidney disease progression, kidney failure, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease (CKD). This approval makes Ozempic the first glucagon-like peptide 1 (GLP-1) receptor agonist indicated for this patient population. This most broadly indicated GLP-1 drug is already approved for cardiovascular events.
Novo also announced expansion of its collaboration with Valo Health to discover and develop novel treatments for obesity, type 2 diabetes, and cardiovascular diseases. This partnership leverages Valo’s extensive human datasets and artificial intelligence capabilities. The expanded agreement is potentially worth US$ 4.6 billion for up to 20 drug programs.
Akero Therapeutics’ stock jumped 100 percent after data from its mid-stage trial showed that 39 percent of patients treated with a 50 mg dose of efruxifermin experienced reversal of cirrhosis with no worsening of metabolic dysfunction-associated steatohepatitis (MASH) after 96 weeks, compared to 15 percent in the placebo group.
Meanwhile, FDA expanded the use of Eli Lilly’s Omvoh (mirikizumab)to include treatment for moderate-to-severe Crohn’s disease, bolstering the company’s immunology portfolio beyond its focus on obesity treatments.
Access the Pipeline Prospector Dashboard for January 2025 Newsmakers (Free Excel)
AbbVie in US$ 1.64 bn molecular glue degraders deal with Neomorph; Galapagos splits into two
AbbVie (stock up 8 percent) and Neomorph entered into a US$ 1.64 billion collaboration to develop molecular glue degraders targeting previously “undruggable” proteins in oncology and immunology. AbbVie also acquired an option to license Simcere Zaiming’s SIM0500, a novel trispecific antibody candidate for multiple myeloma, in a deal valued at up to US$ 1.06 billion. SIM0500 is currently in early-stage clinical trials in both China and the US.
Boehringer Ingelheim has expanded its oncology pipeline with two licensing deals. First, the company partnered Synaffix in a deal worth up to US$ 1.3 billion to advance antibody-drug conjugates (ADCs). Second, it exercised the option to gain rights to a fourth novel cancer target from an ongoing discovery collaboration with Oxford BioTherapeutics, which began in 2013.
Sanofi (stock up 10 percent) announced plans to buy back € 5 billion (US$ 5.21 billion) in shares in 2025, signaling potential increases in acquisition activities following the sale of its Opella consumer health unit. This divestment marks Sanofi’s shift to focusing exclusively on drugs and vaccines.
Galapagos (stock down 18 percent) announced plans to split into two distinct publicly traded entities. The entity that retains the Galapagos name will focus on advancing its cell therapy programs in oncology, while the other one will focus on developing a pipeline of innovative medicines through strategic transactions. It will be capitalized with approximately € 2.45 billion (US$ 2.53 billion) from Galapagos’ current cash reserves.
Access the Pipeline Prospector Dashboard for January 2025 Newsmakers (Free Excel)
J&J’s Spravato approved for depression by FDA; another Astra-Daiichi ADC sees light of day
The psychedelic medicine frontier showed promise with J&J’s Spravato (esketamine) achieving a breakthrough as FDA approved it for treatment-resistant depression (standalone treatment). The nasal spray demonstrated impressive efficacy, with 22.5 percent of patients achieving remission by week four.
Atai Life Sciences also announced encouraging results from a mid-phase study evaluating BPL-003, an intranasal formulation of 5-MeO-DMT benzoate in patients with moderate to severe alcohol use disorder (AUD).
In oncology, AstraZeneca (stock up 9 percent) and Daiichi Sankyo (stock up 3 percent) secured US approval for Datroway (datopotamab deruxtecan) for treating unresectable or metastatic hormone receptor-positive, HER2-negative breast cancer. Meanwhile, Amgen’s combination therapy of Lumakras (sotorasib) with Vectibix (panitumumab) received FDA approval for KRAS G12C-mutated metastatic colorectal cancer. Amgen’s stock gained 9 percent.
FDA approved Vertex Pharmaceuticals’ Journavx (suzetrigine), a first-in-class non-opioid oral pain signal inhibitor. This approval marks the first new class of pain medicine in over 20 years, offering an effective alternative without addictive potential. The US agency also approved Axsome Therapeutics’ rapid-acting Symbravo (meloxicam and rizatriptan) for the acute treatment of migraine.
Access the Pipeline Prospector Dashboard for January 2025 Newsmakers (Free Excel)
Our view
If 2024 ended with a spate of drug approvals, 2025 began with both increased dealmaking and approvals. However, post January 20, the world is a different place. Trump administration’s tariff policies have spurred a trade war, and China has retaliated with tariffs of US coal and gas.
At present, there is little certainty on what the tariffs mean for different pharma companies, especially those who don’t manufacture in the US. While some drugmakers are hopeful that Trump will crack down on the pharmacy benefit managers, others are more concerned on what higher tariffs on EU and Indian drugmakers would mean for generic drug prices. We expect volatility for at least some months.
Access the Pipeline Prospector Dashboard for January 2025 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in January 2025
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : INDICES SPIKE AMID DEAL BONANZA IN JAN by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







