By PharmaCompass
2024-07-04
Impressions: 2850
The pharma indices were back in the black in May, and the good streak continued through June with the Nasdaq Biotechnology Index (NBI) gaining 3 percent, the SPDR S&P Biotech ETF (XBI) index up over 3.1 percent and the S&P Biotechnology Select Industry Index (SPSIBI) rising 4.25 percent.
The two summer months were similar in more ways than one. In May, Eli Lilly had announced an investment of US$ 5.3 billion to boost the supply of Zepbound and Mounjaro. In June, Novo Nordisk followed suit, investing US$ 4.1 billion to develop a new manufacturing facility to boost the supply of Ozempic and Wegovy.
Similarly, the US Food and Drug Administration (FDA) continued to grant vaccine approvals in June after okaying Moderna’s mRNA respiratory syncytial virus (RSV) vaccine in May. Last month, Merck’s next-generation pneumococcal vaccine won an FDA nod, and the agency expanded the use of GSK’s respiratory syncytial virus (RSV) vaccine to include adults aged 50 to 59.
However, June was a lackluster month for mergers and acquisitions. The month had to be content with some tie-ups. For instance, Roche signed an up to US$ 1.8 billion deal with Boston-based startup Ascidian Therapeutics to discover and develop novel gene therapies for difficult-to-treat neurological diseases. AbbVie inked a US$ 1.7 billion agreement with China’s FutureGen to bring the latter’s next-generation treatment (FG-M701) for inflammatory bowel disease to market. Takeda signed an option agreement with China’s Ascentage Pharma for an exclusive license to a promising drug for chronic myeloid leukemia and other blood cancers. And Day One Biopharmaceuticals struck a licensing deal with MabCare for a novel antibody drug conjugate (ADC) to treat multiple cancers. The deal’s potential value is US$ 1.2 billion.
Access the Pipeline Prospector Dashboard for June 2024 Newsmakers (Free Excel)
Merck’s pneumococcal jab wins FDA nod; Moderna’s combo vaccine scores trial win
Vaccines took centerstage in June. FDA approved Merck’s Capvaxive, a next-generation pneumococcal vaccine designed to protect adults from a broader range of pneumococcus bacteria strains that cause serious illnesses and pneumonia. Capvaxive targets 21 bacterial variations responsible for about 85 percent of invasive pneumococcal disease cases in older adults.
Similarly, GSK secured FDA approval for its RSV vaccine for adults aged 50 to 59 years. This makes Arexvy the only RSV shot endorsed for that age group.
In a late-stage trial, Moderna’s mRNA-1083, an investigational combination vaccine against influenza and Covid, elicited a higher immune response compared to separate shots in people aged 50 and over. The combo jab generated more antibodies than currently marketed flu vaccines and Moderna’s Spikevax. And the world’s first personalized mRNA cancer vaccine from Moderna has raised hopes for patients with skin cancer, as three-year data from a mid-stage trial showed some benefits on patients who took the vaccine in combination with Merck’s Keytruda.
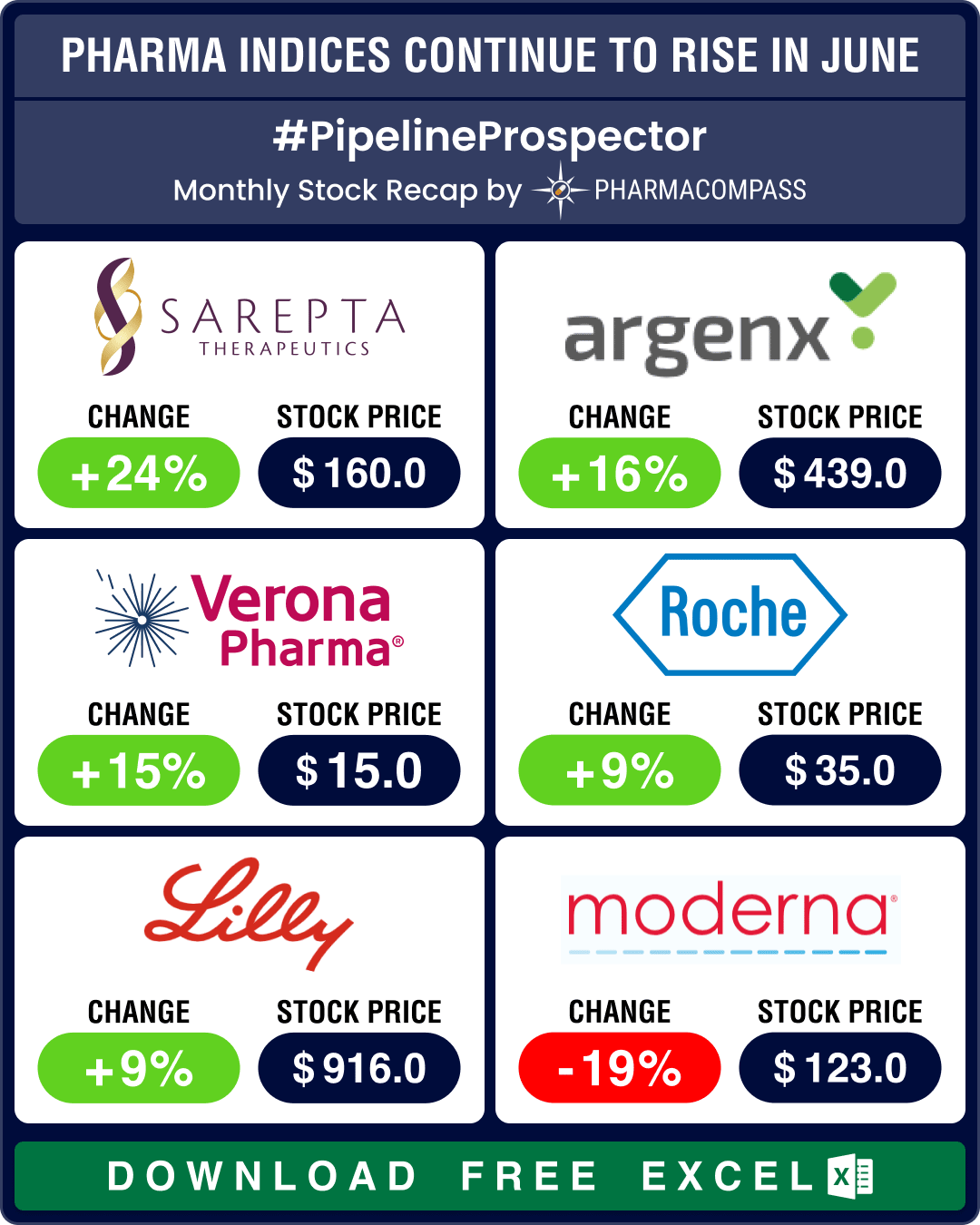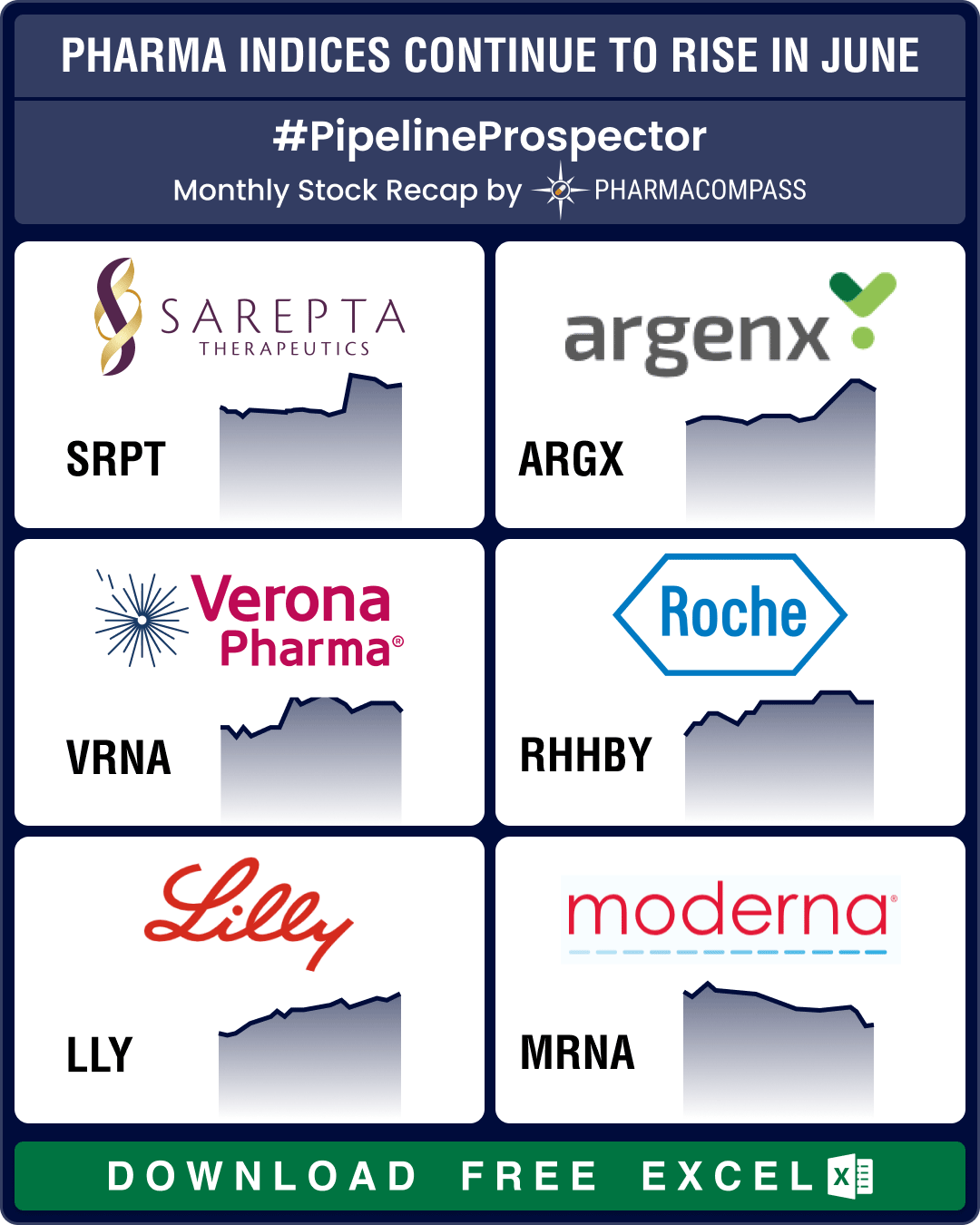
Despite these developments, Moderna’s stock dropped 19 percent in June after it said the efficacy of its RSV vaccine mRESVIA had vaned substantially to 50 percent after 18 months.
Access the Pipeline Prospector Dashboard for June 2024 Newsmakers (Free Excel)
Verona, Geron score maiden FDA approvals; BMS’ Augtyro bags tumor-agnostic nod
Two companies obtained their first FDA approvals last month. The first one was Verona Pharma (stock up 15 percent) — it received its maiden FDA approval for Ohtuvayre for treating chronic obstructive pulmonary disease (COPD). This is also the first inhaled COPD treatment with a novel mechanism of action.
The second company to get its maiden FDA approval was Geron, a commercial-stage biopharmaceutical company. FDA signed off on Geron’s Rytelo for treating transfusion-dependent anemia in patients with low- to intermediate-risk myelodysplastic syndromes (MDS), a group of blood cancers.
In June, FDA granted accelerated approvals to at least three drugs. BMS’ Augtyro was granted FDA’s accelerated approval for treating adult and pediatric patients (over 12 years) with solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, regardless of where they occur in the body.
Ipsen received FDA’s accelerated approval for Iqirvo, a first-in-class, once-daily oral medication for treating primary biliary cholangitis (PBC), a progressive liver disease affecting mostly women.
Sarepta Therapeutics’ (stock up 24 percent) Elevidys received accelerated approval for Duchenne muscular dystrophy (DMD) despite failing its primary endpoint in a late-stage trial.
Amongst other approvals, Argenx’s Vyvgart Hytrulo was approved to treat chronic inflammatory demyelinating polyneuropathy (CIDP). This is a novel treatment option for this rare and debilitating neuromuscular disorder. And BMS’ Krazati got its second approval for treating colorectal cancer with a specific KRAS mutation.
Access the Pipeline Prospector Dashboard for June 2024 Newsmakers (Free Excel)
GSK’s failed cancer drug posts trial win; Gilead’s HIV prophylaxis shows efficacy
In late-stage trials, GSK’s multiple myeloma therapy Blenrep cut the risk of disease progression or death by almost half compared to standard-of-care treatments. The once-failed ADC was pulled from the lucrative US market in 2022, but the results could signal a comeback for Blenrep.
Intra-Cellular’s antipsychotic drug Caplyta scored another remarkable late-stage win for treating major depressive disorder (MDD).
In another late-stage trial, Gilead’s (stock up 6 percent) long-acting injection proved to be more effective in preventing HIV infection in women compared to the daily pill Truvada. This is the first time that an HIV pre-exposure prophylaxis (PrEP) has shown zero infections in a phase 3 trial, the drugmaker said. Dosed just twice a year, Sunlenca could be a game-changer in HIV prevention. Analysts estimate that PrEP-related sales could be over US$ 1.7 billion.
In a blow to millions affected by long Covid, Pfizer’s Paxlovid did not appear to improve the symptoms as was hoped. Pfizer’s stock fell 7 percent in June.
Access the Pipeline Prospector Dashboard for June 2024 Newsmakers (Free Excel)
Our view
This was the second month when pharma indices showed an upward trend. It’s been a good first half for the US stock markets. But there are mixed reports on how markets will perform in the second half, given the uncertainties around interest rate cuts and the outcome of the presidential elections in the US slated to be held in November. Though for those tracking biopharma news, there are enough interesting developments to fret over the markets.
Access the Pipeline Prospector Dashboard for June 2024 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in Jun 2024
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Pharma & Biotech Newsmakers in Jun 2024 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







