Stock Recap #PipelineProspector
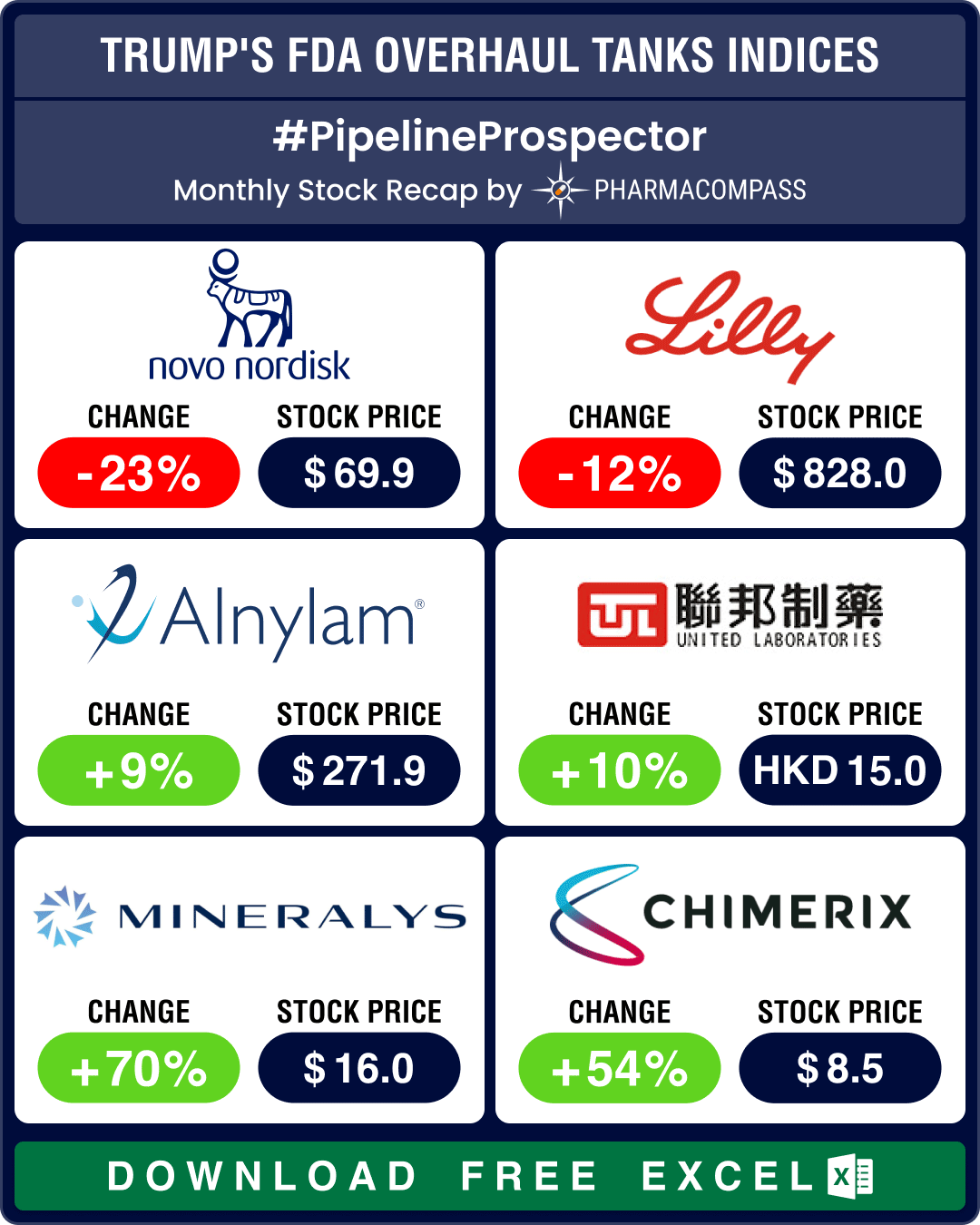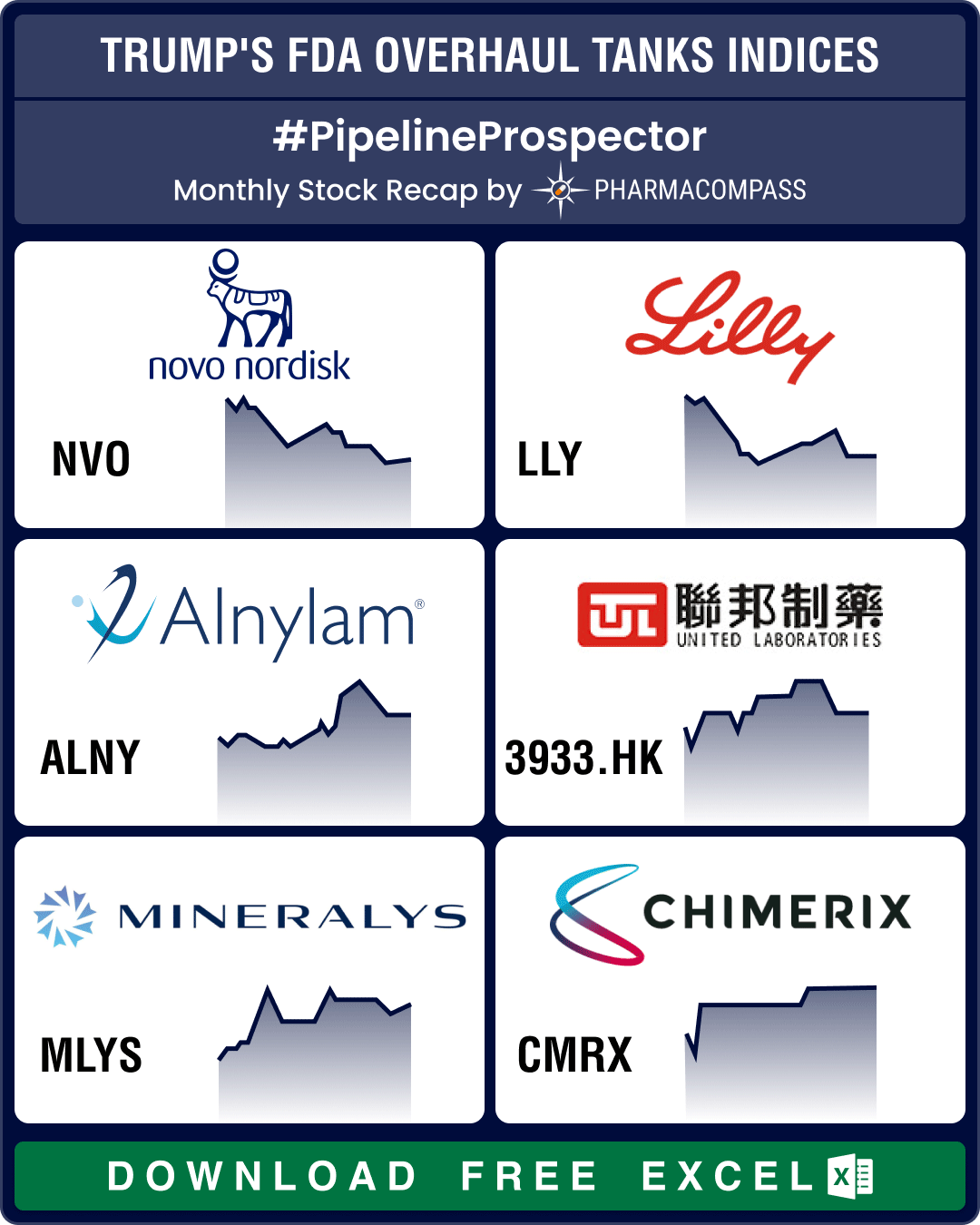
Pipeline Prospector March 2025: Trump’s FDA overhaul spooks biotech stocks; Roche, AbbVie, Novo ink obesity drug deals
March ended with news that the top vaccine regulator of the US
Food and Drug Administration (FDA),
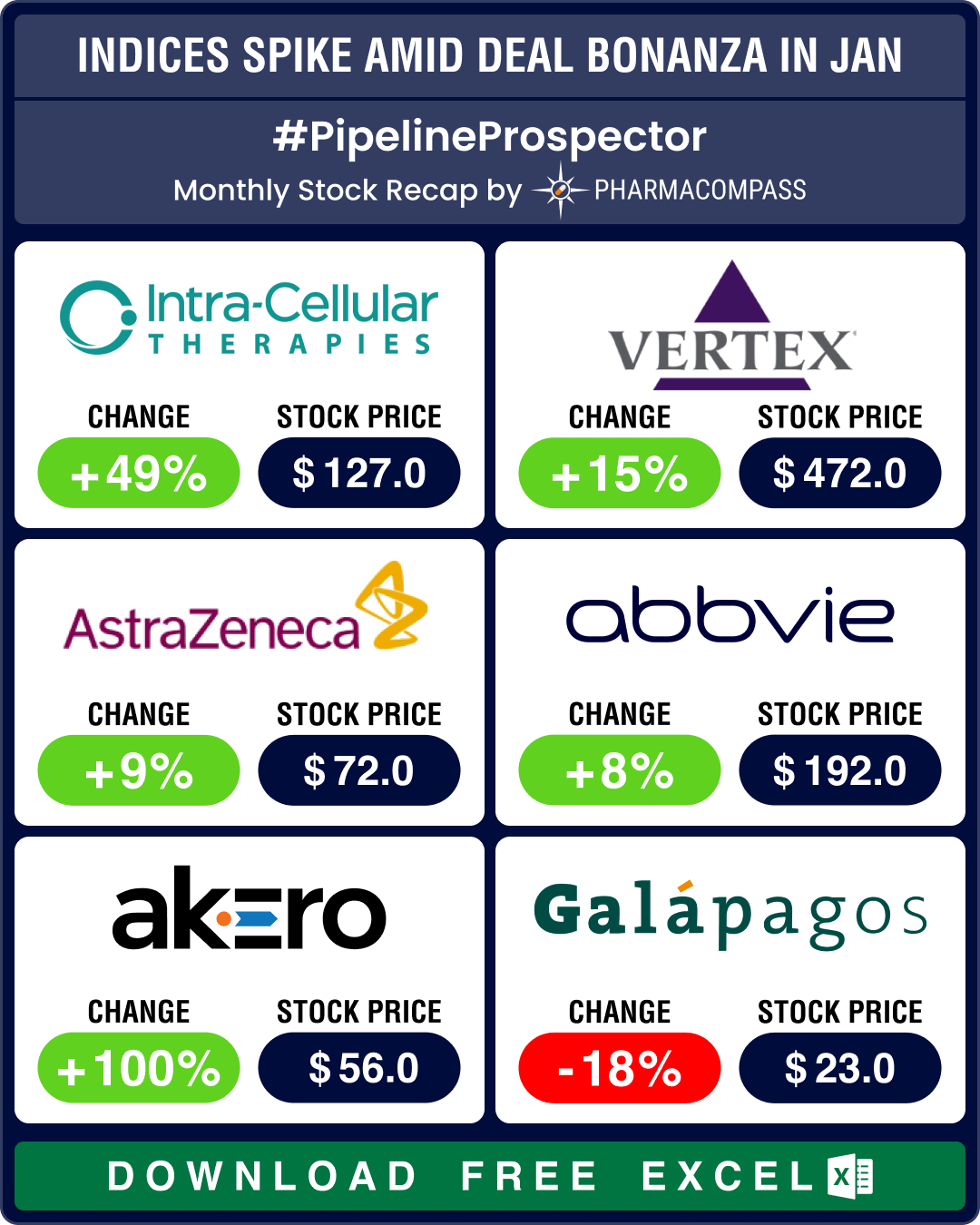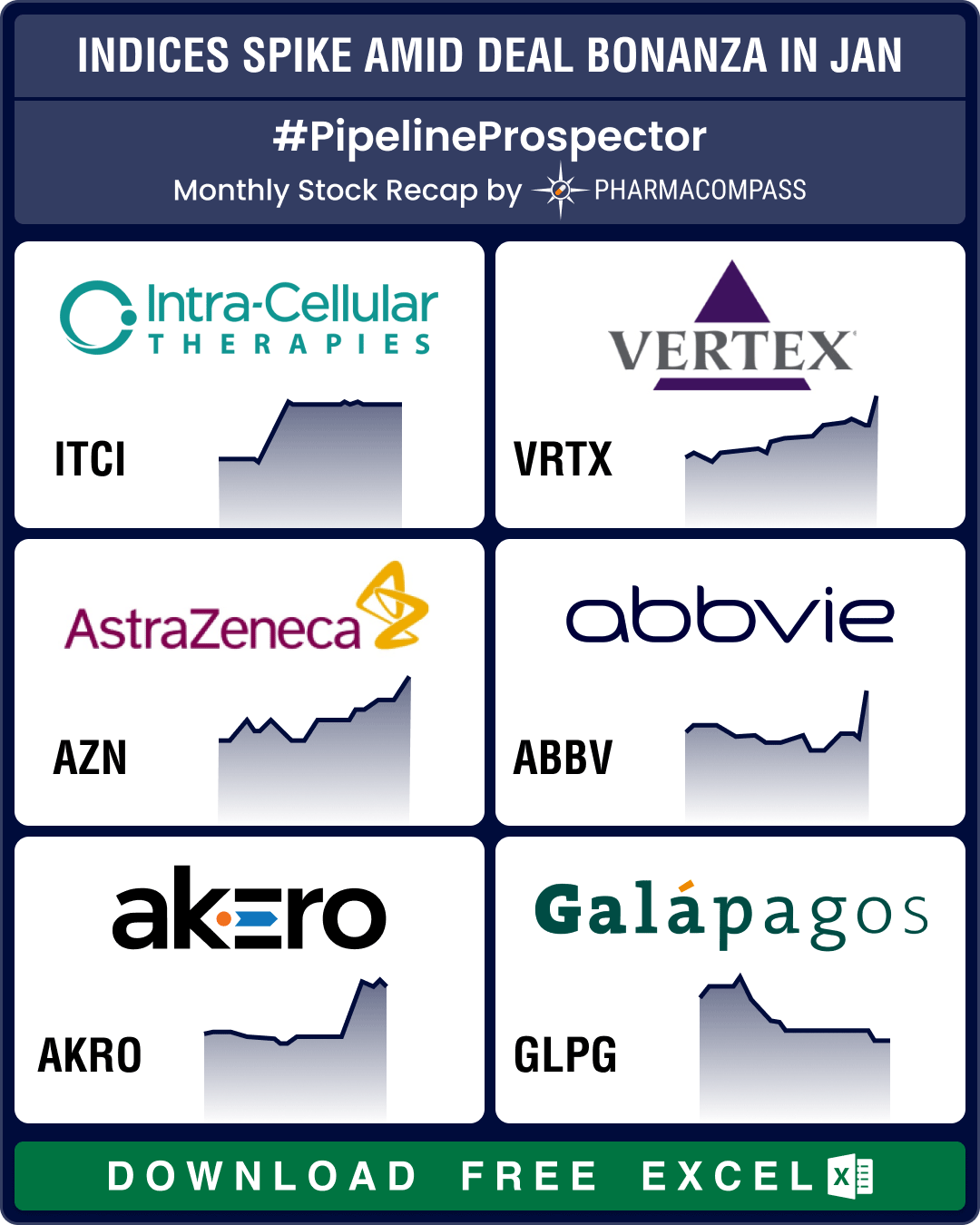
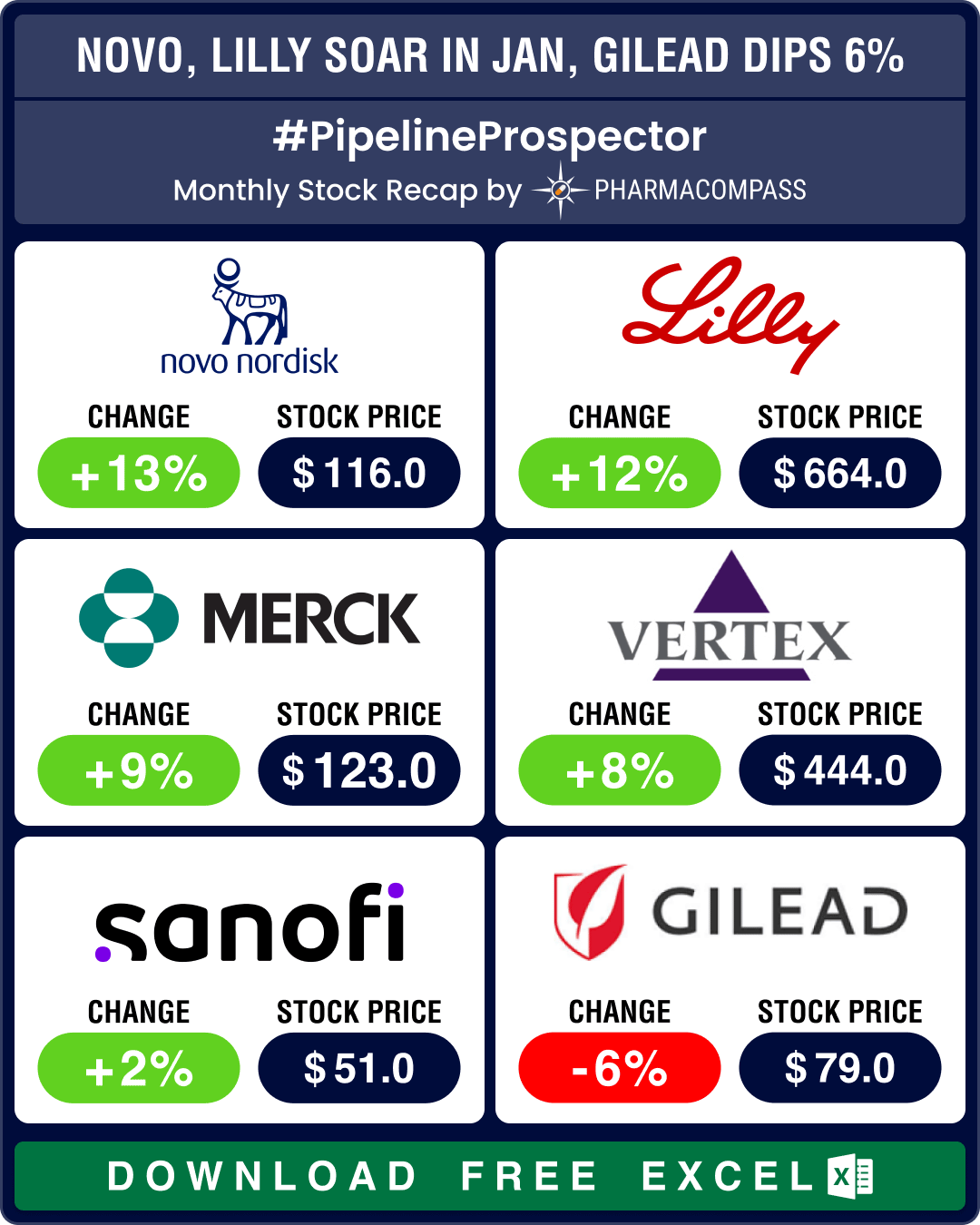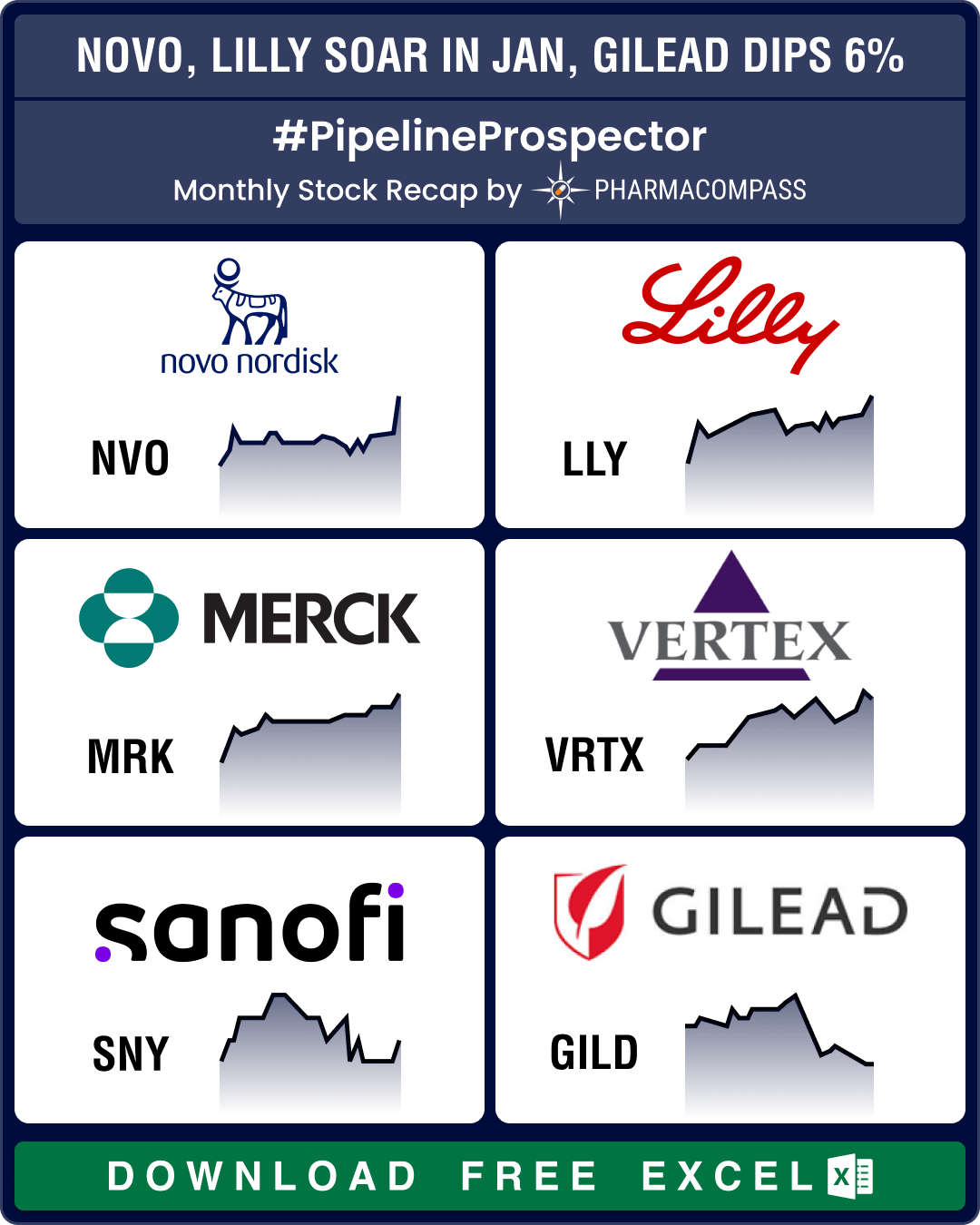
Pipeline Prospector Jan 2025: J&J’s US$ 14.6 bn Intra-Cellular buyout kicks off deal frenzy; Ozempic clinches FDA nod for CKD
January was a busy month that saw several deals being announced at the JP Morgan Healthcare Conferen
Pipeline Prospector 2024 highlights: Rise in new breed of biotechs with maiden approvals; GLP-1 meds show promise beyond obesity
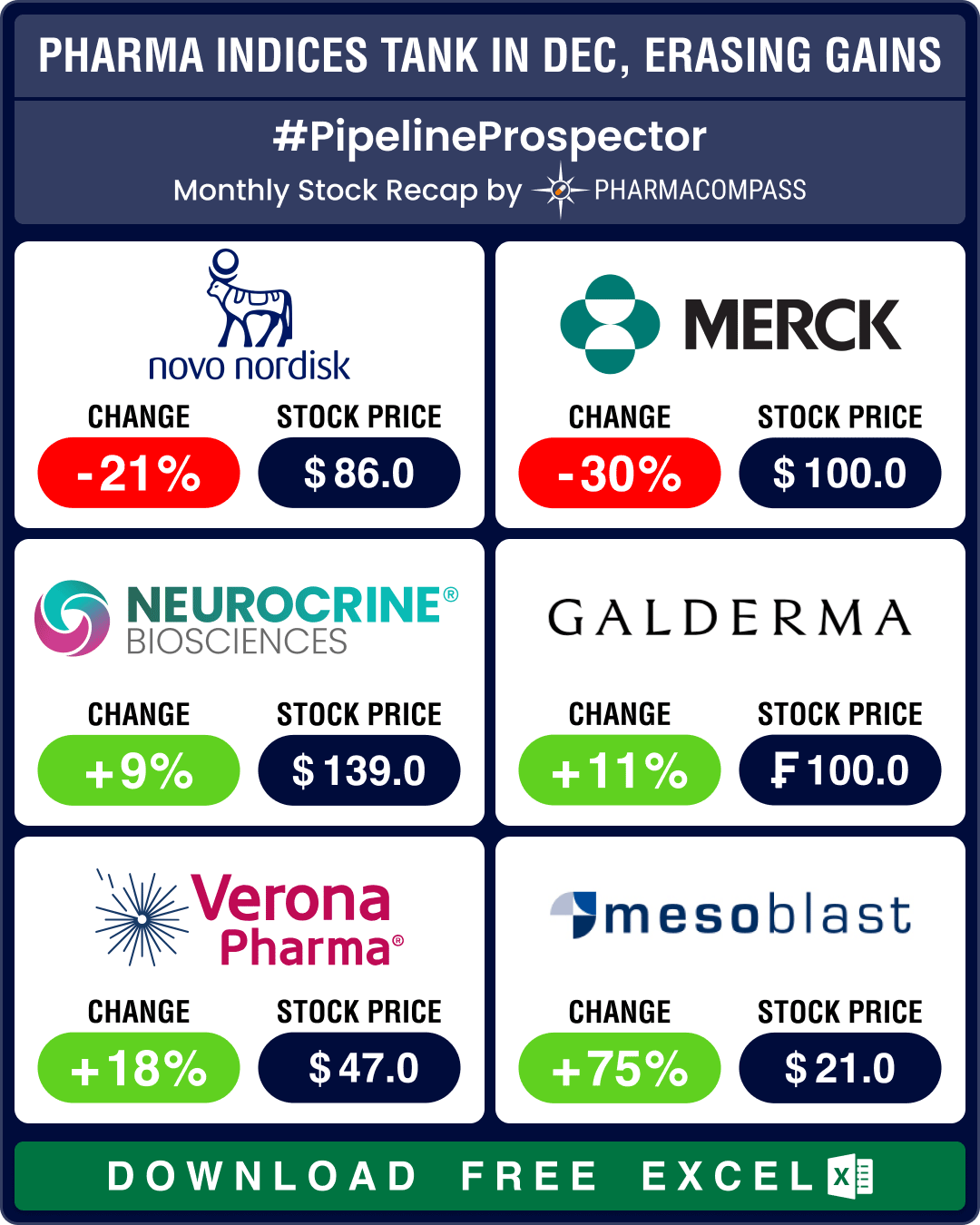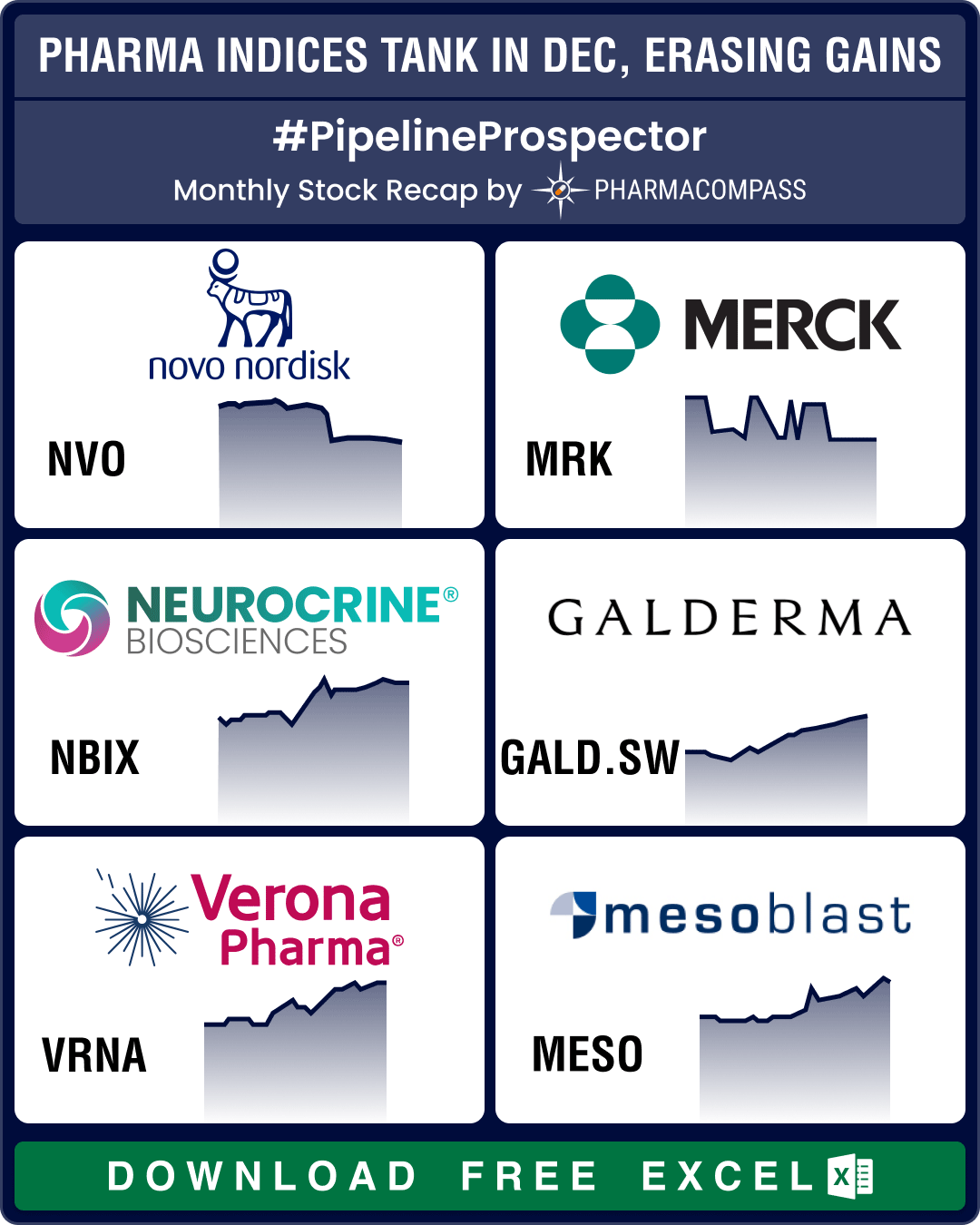
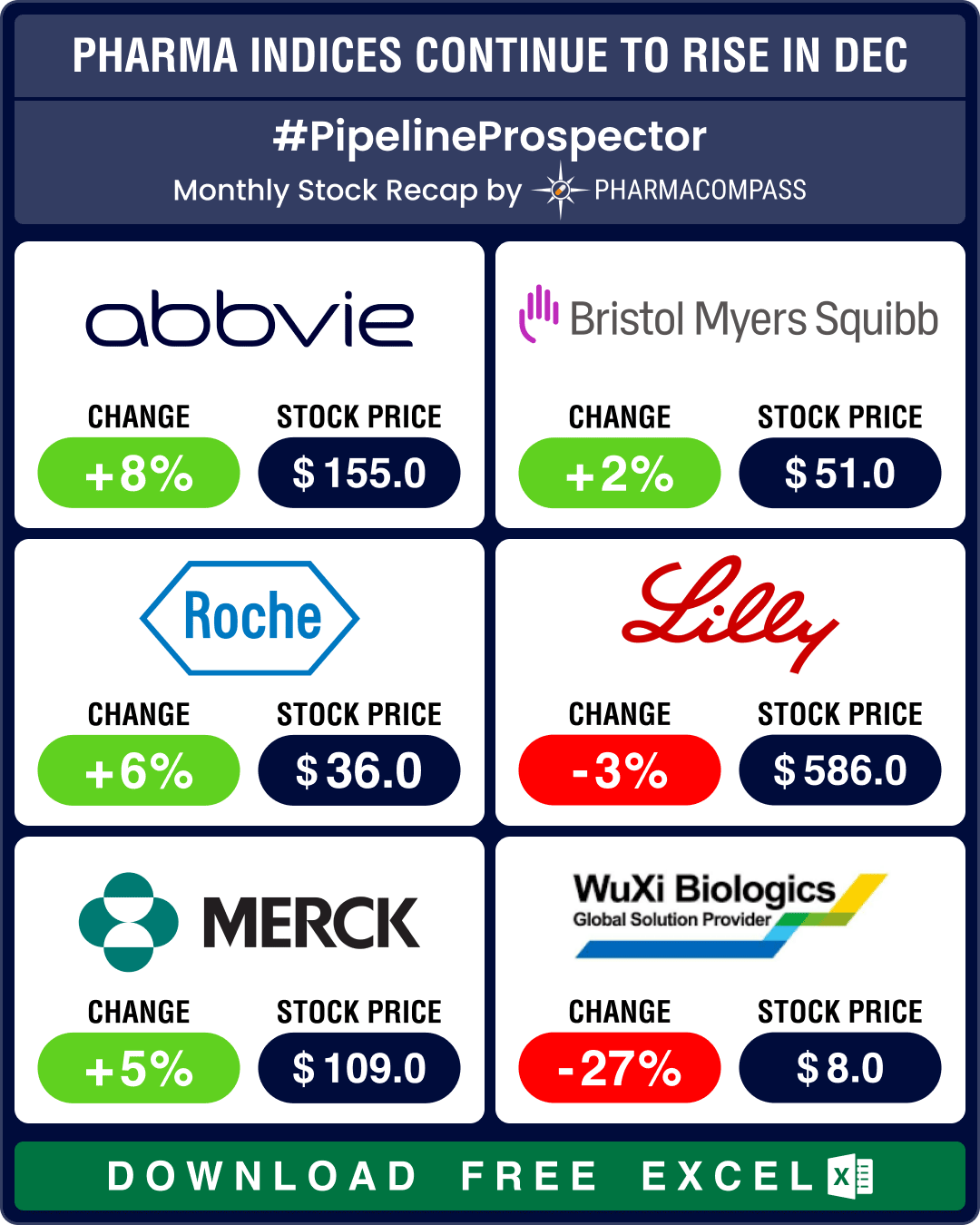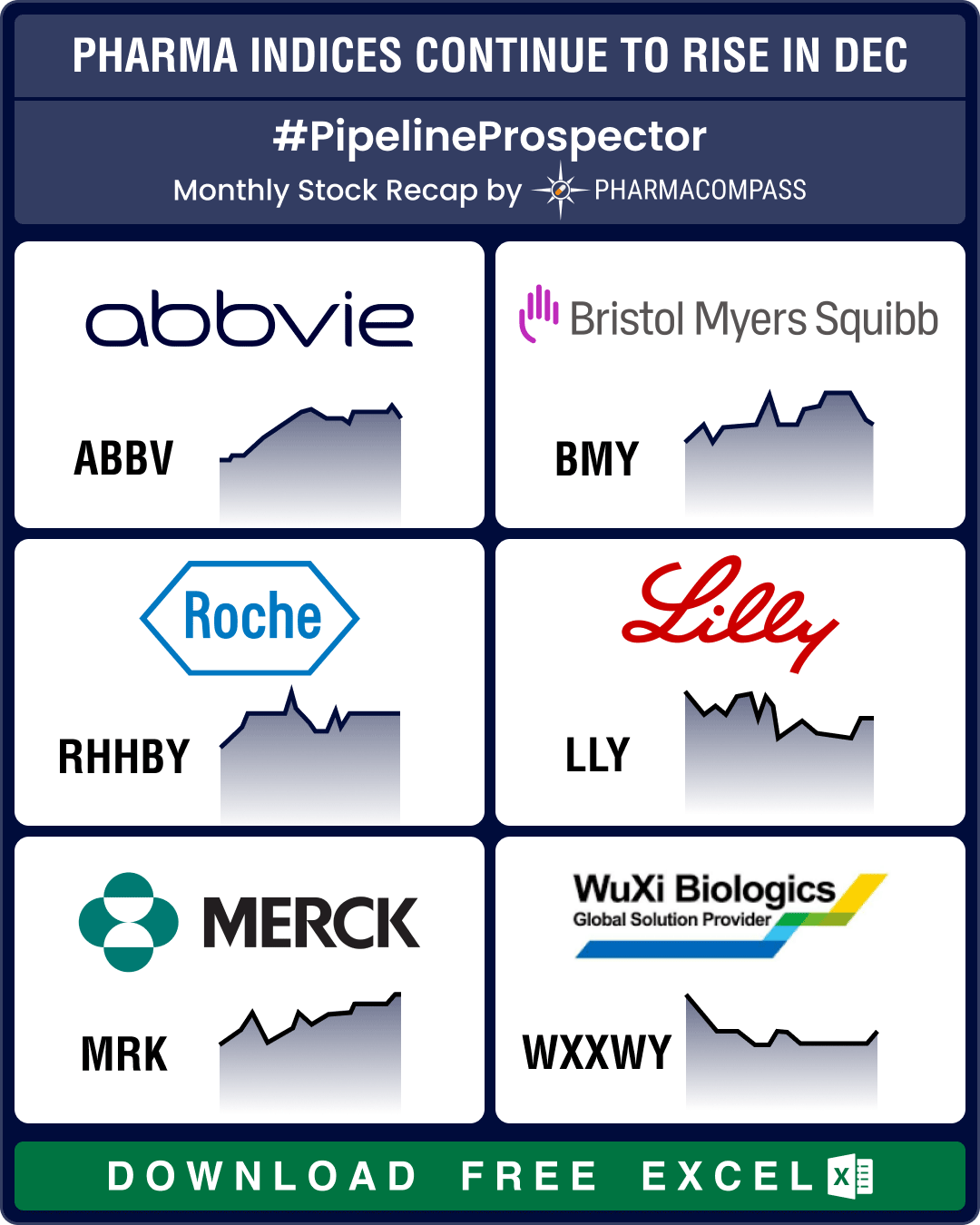
December proved to be one of the most bearish months of 2024 for the biopharma sector. The Nasdaq Bi
Pipeline Prospector July 2024: Indices continue to climb; Lilly buys Morphic for US$ 3.2 bn, Kisunla bags FDA nod
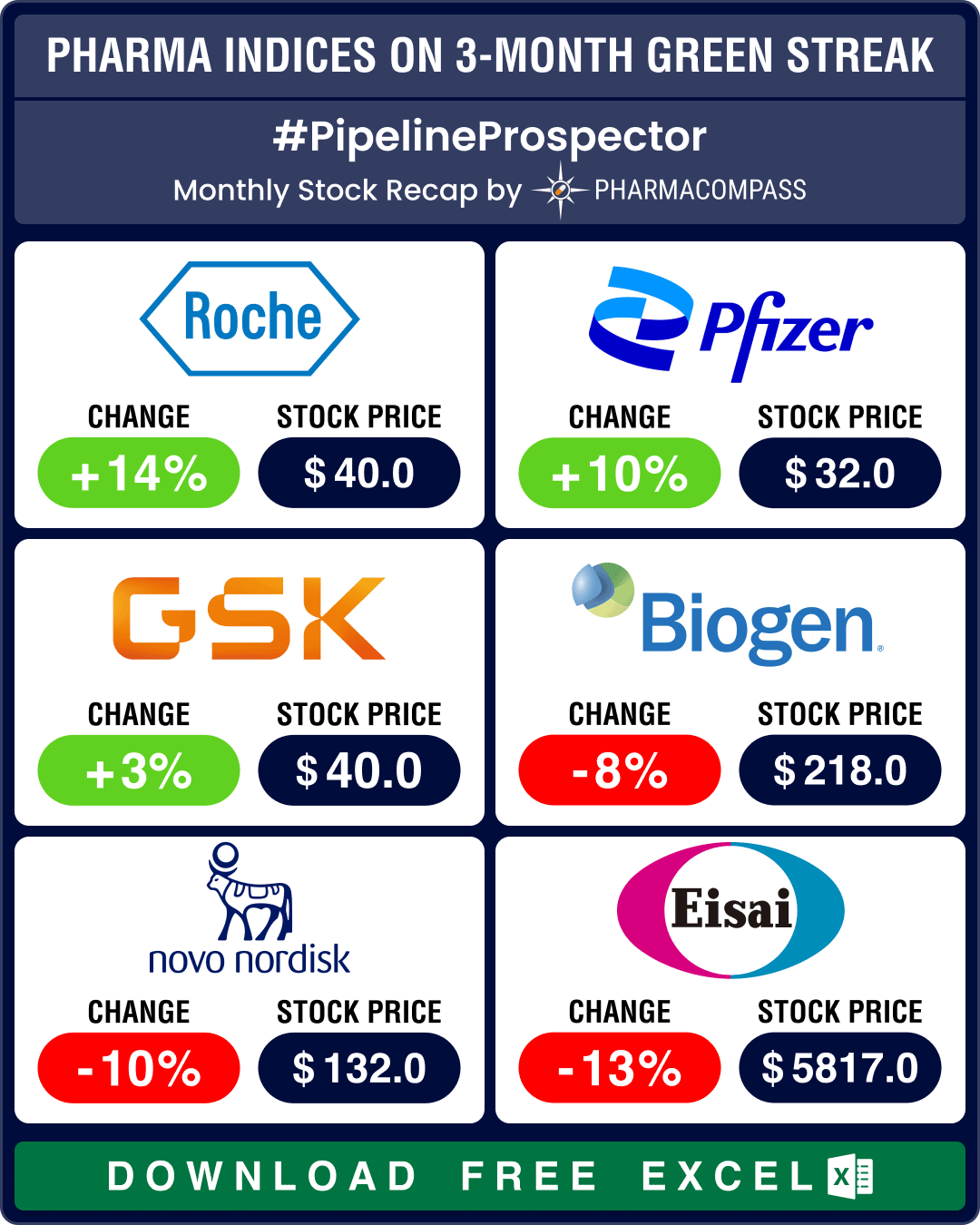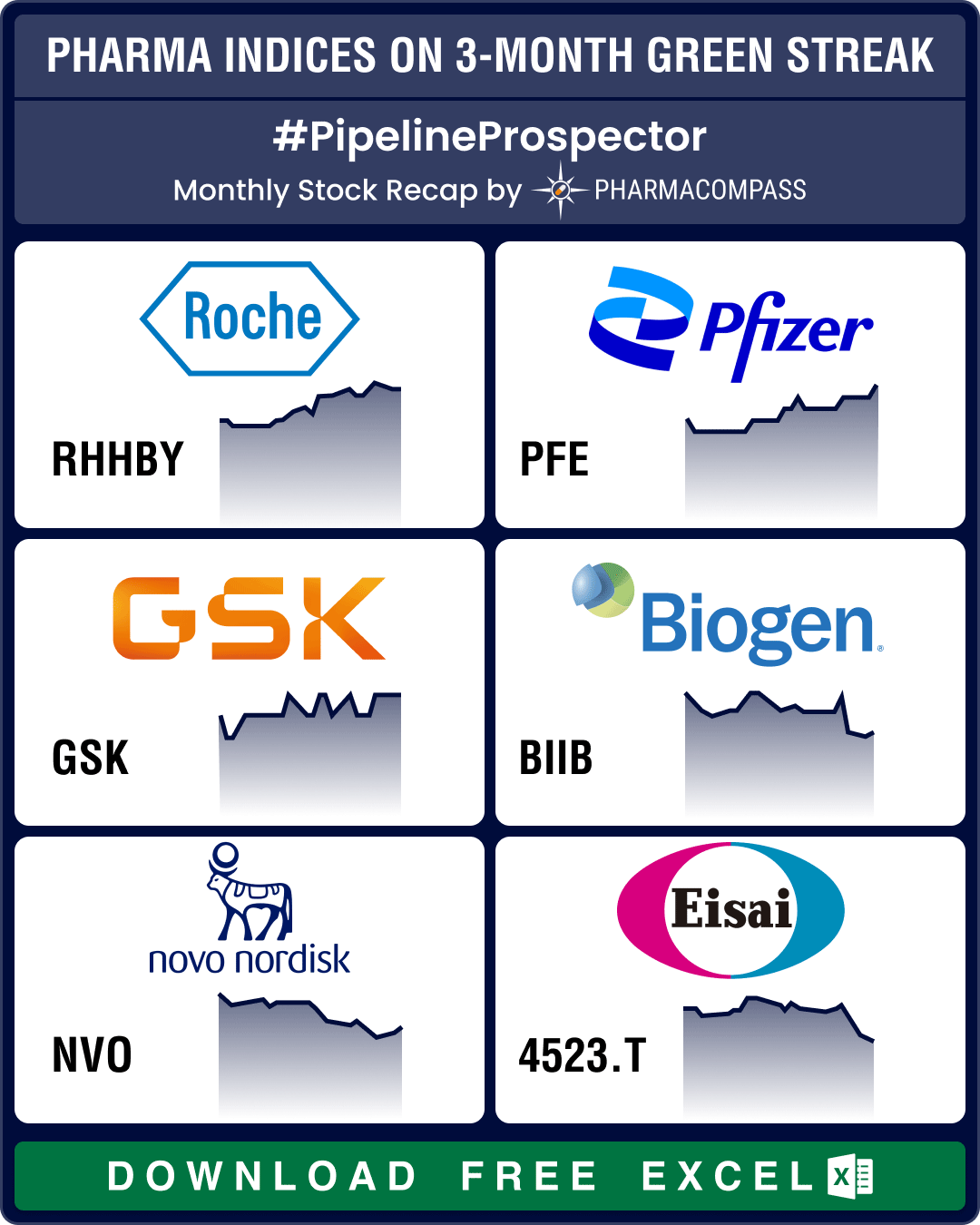
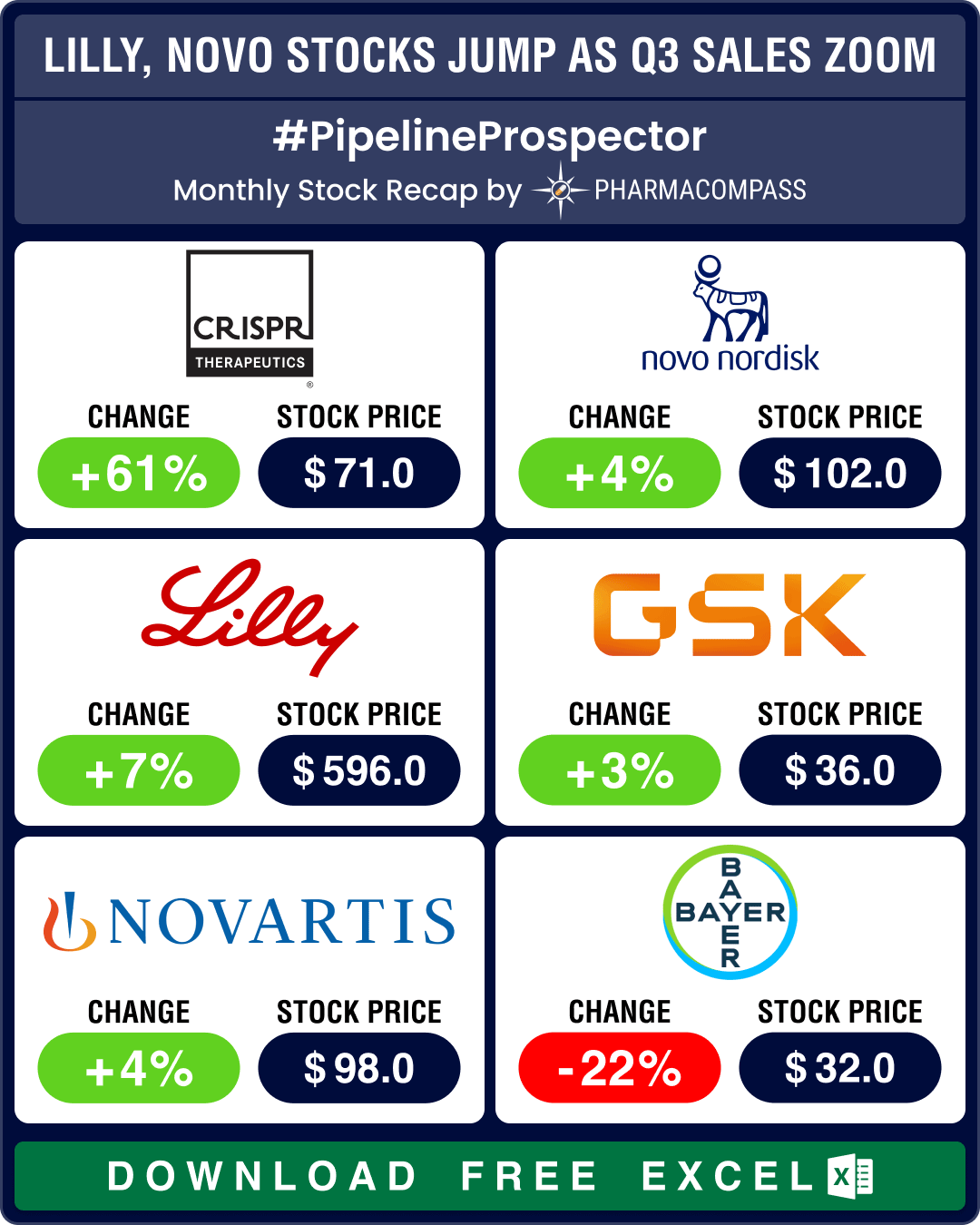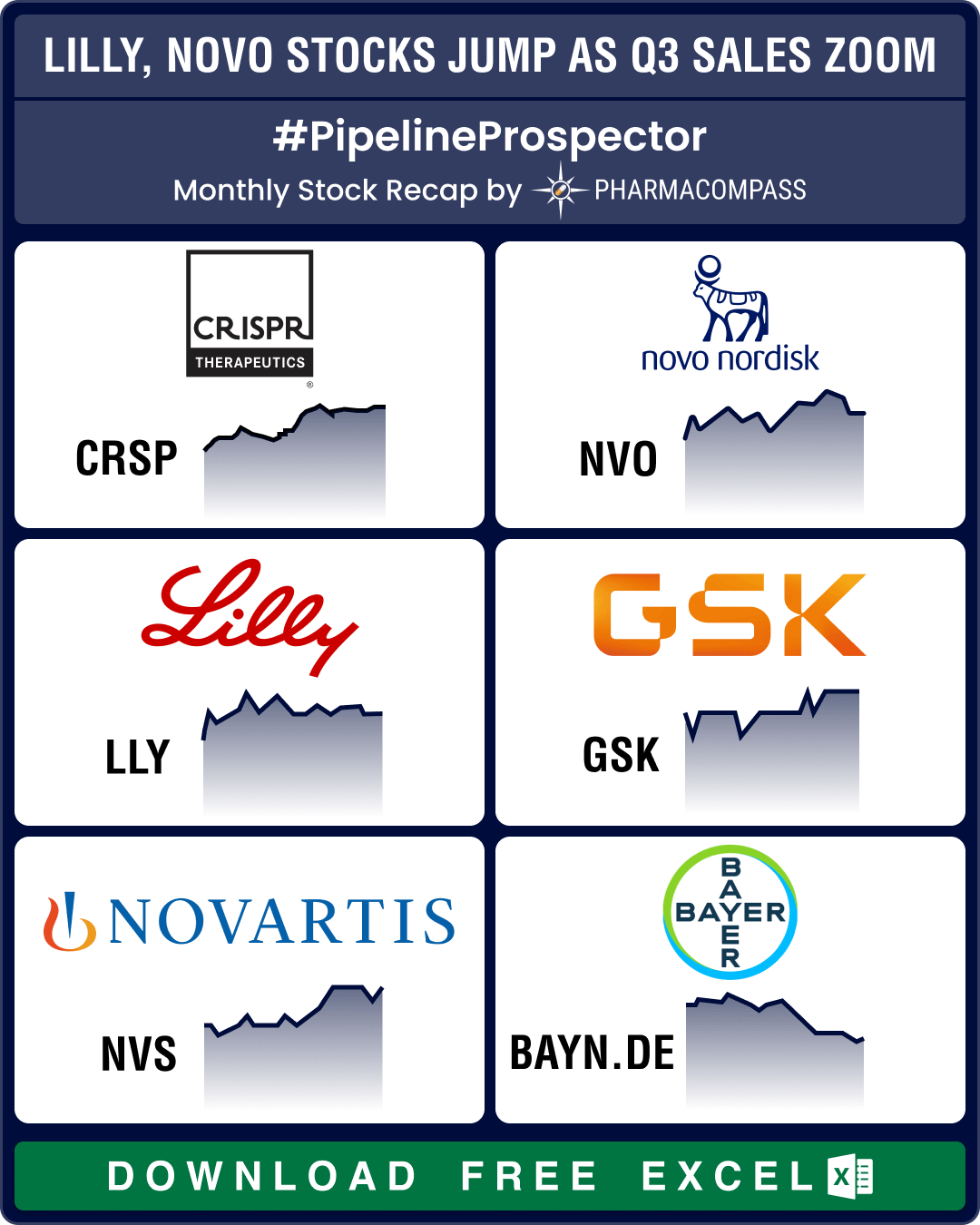
The biotechnology sector ended in the green for the third month in a row in July, significantly outp
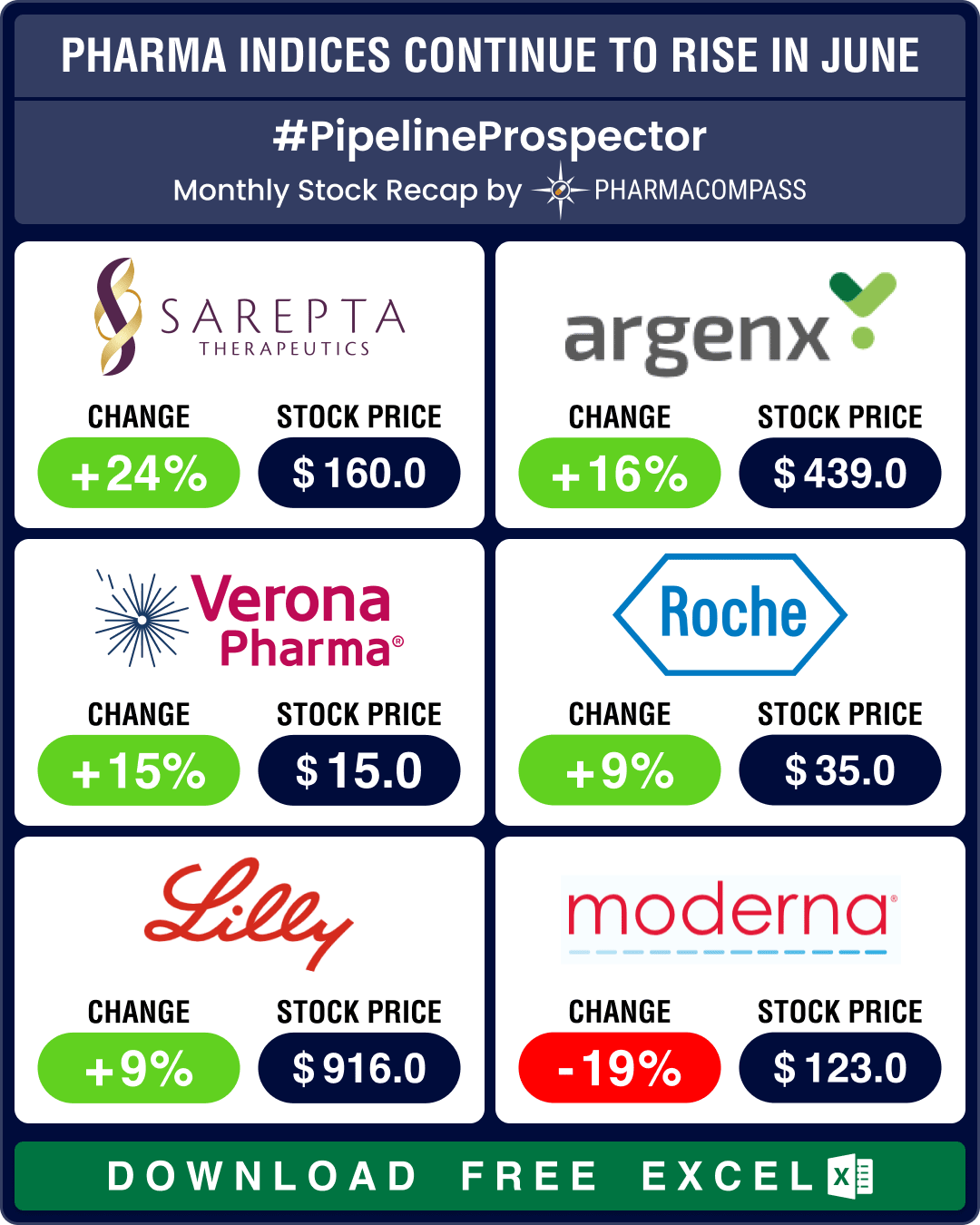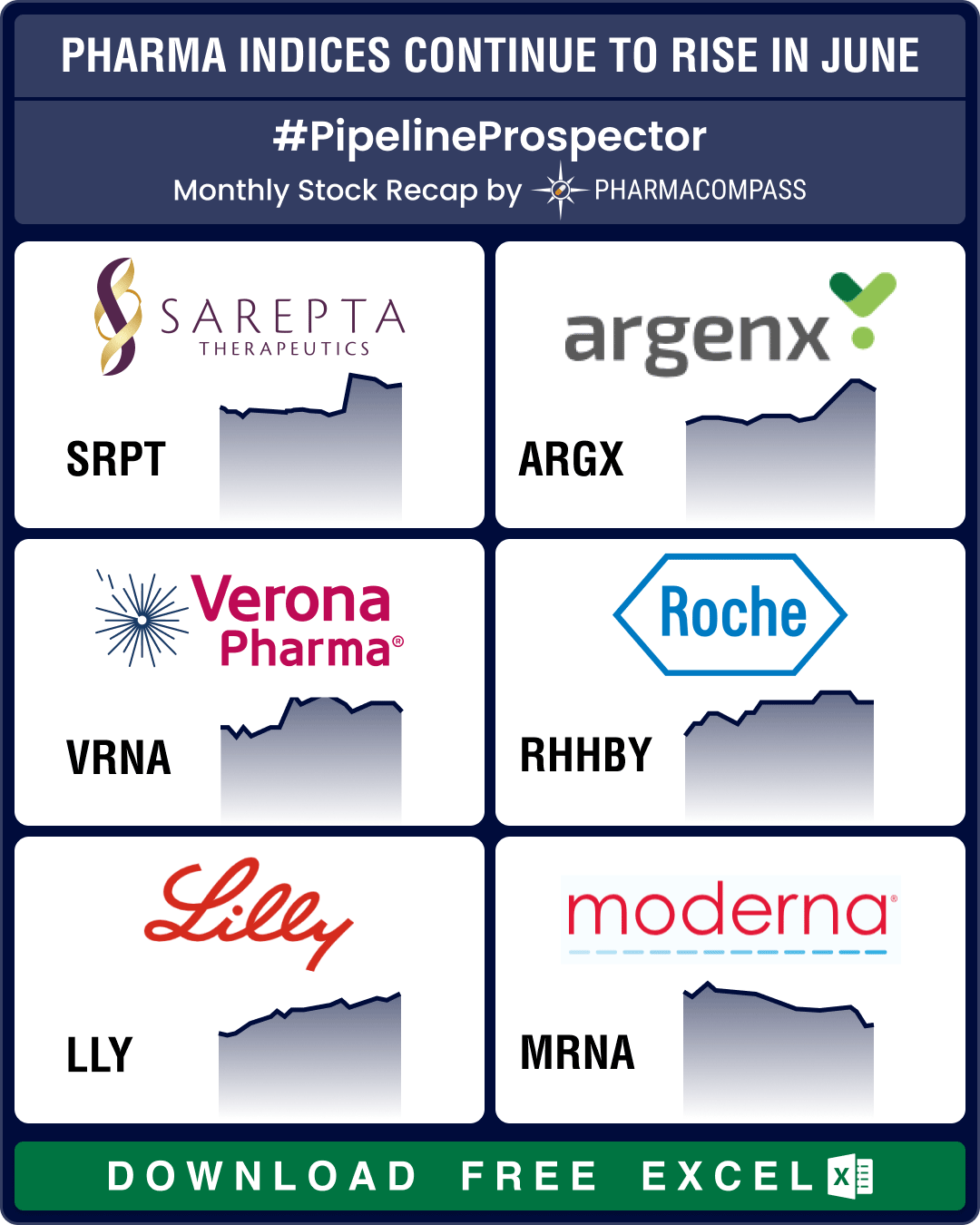
Pipeline Prospector June 2024: FDA approves Merck’s next-gen pneumococcal vaccine, Verona’s COPD therapy
The pharma indices were back in the black in May, and the good streak continued through June with th
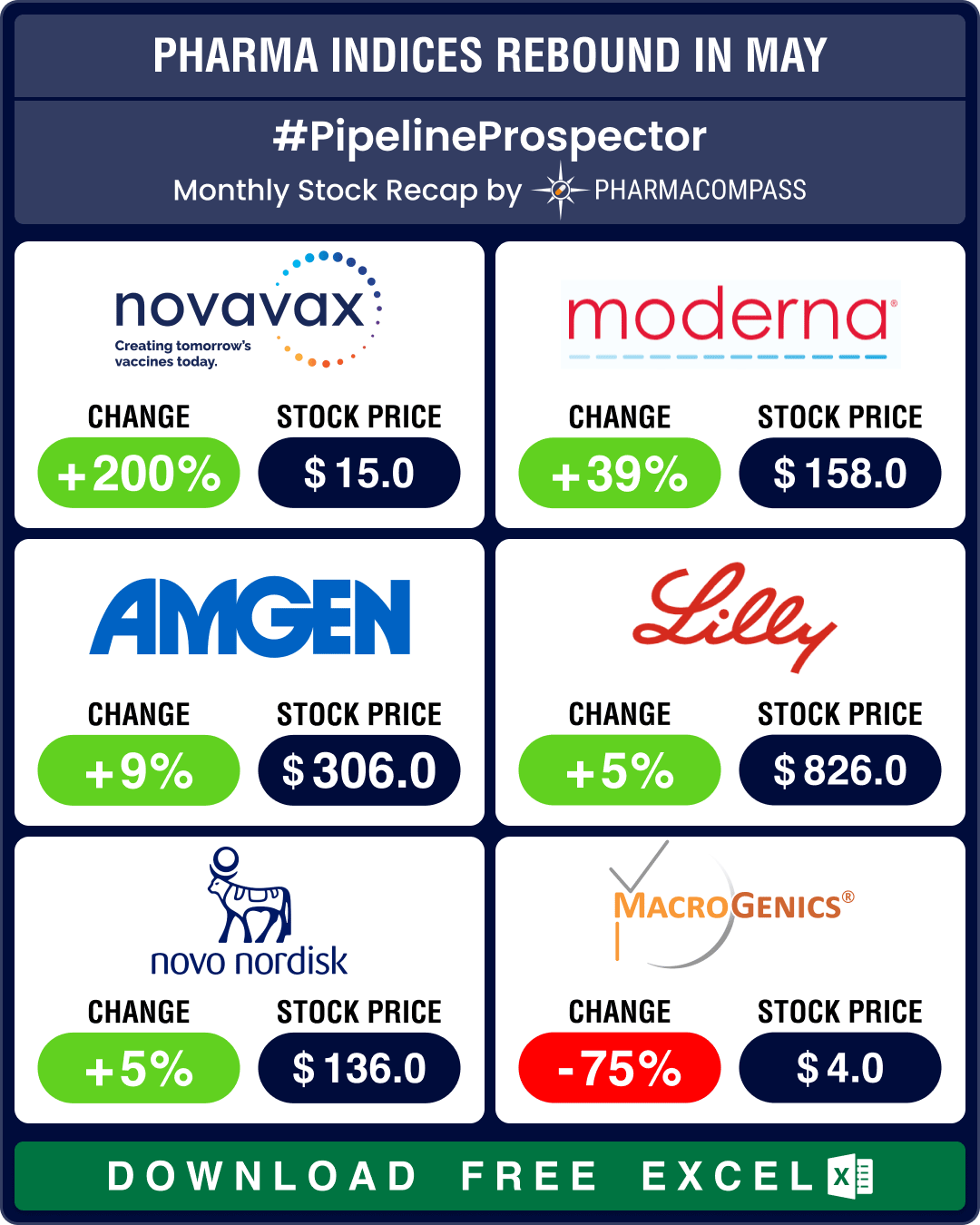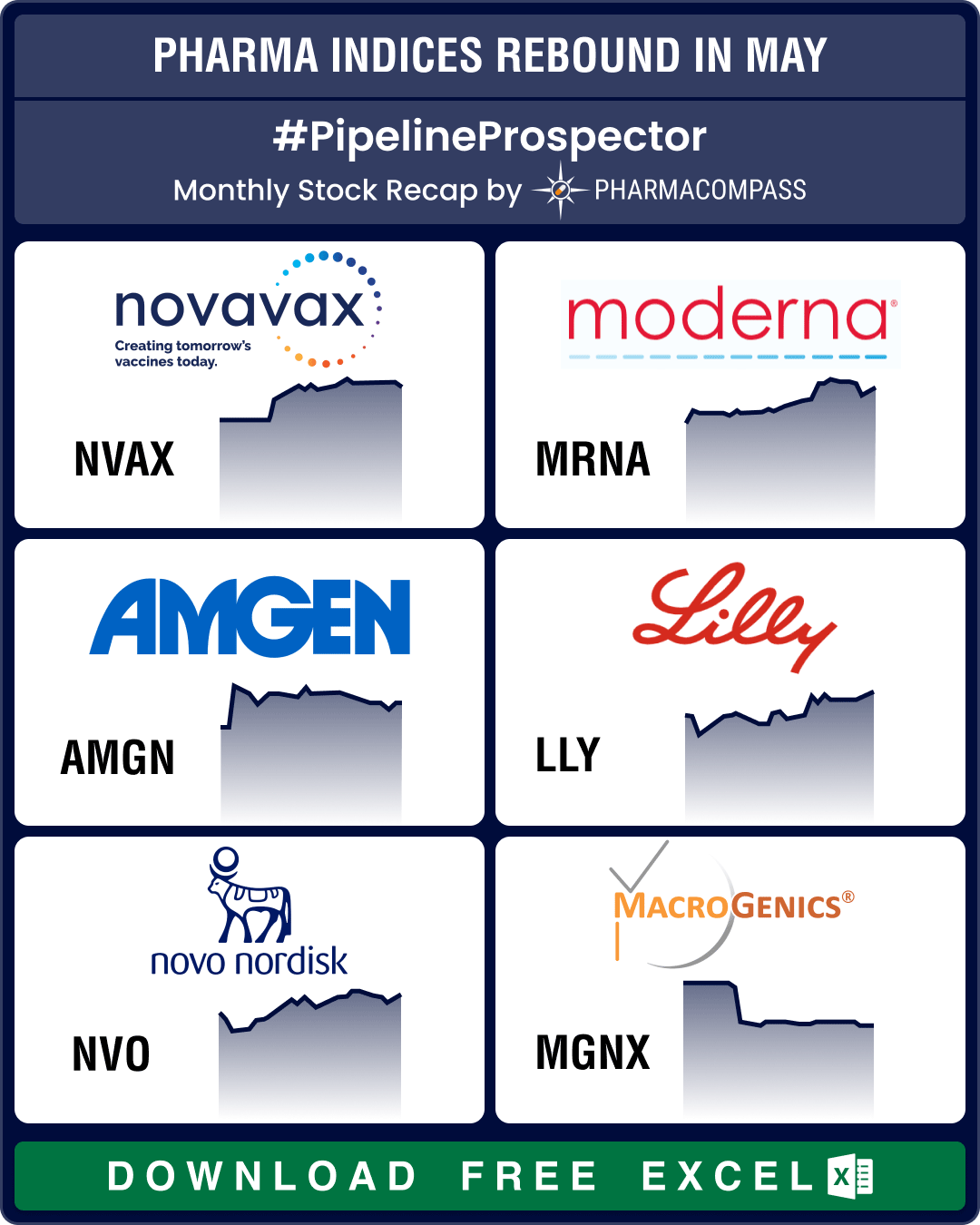
Pipeline Prospector May 2024: J&J inks two deals for eczema drugs; Novo scores trial wins in hemophilia, kidney disease
Pharma indices have rebounded after ending March and April in the red. May saw the Nasdaq Biotechnol
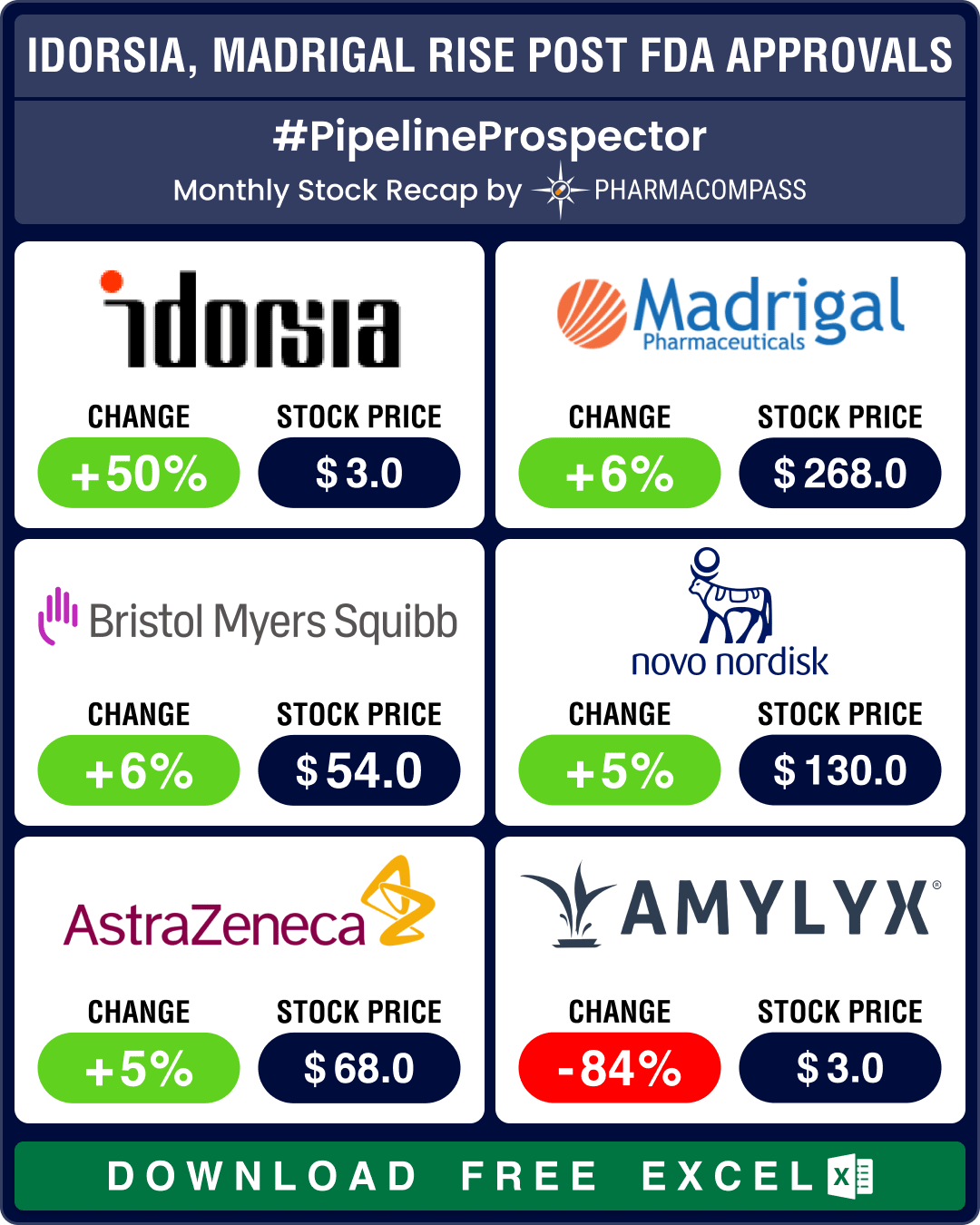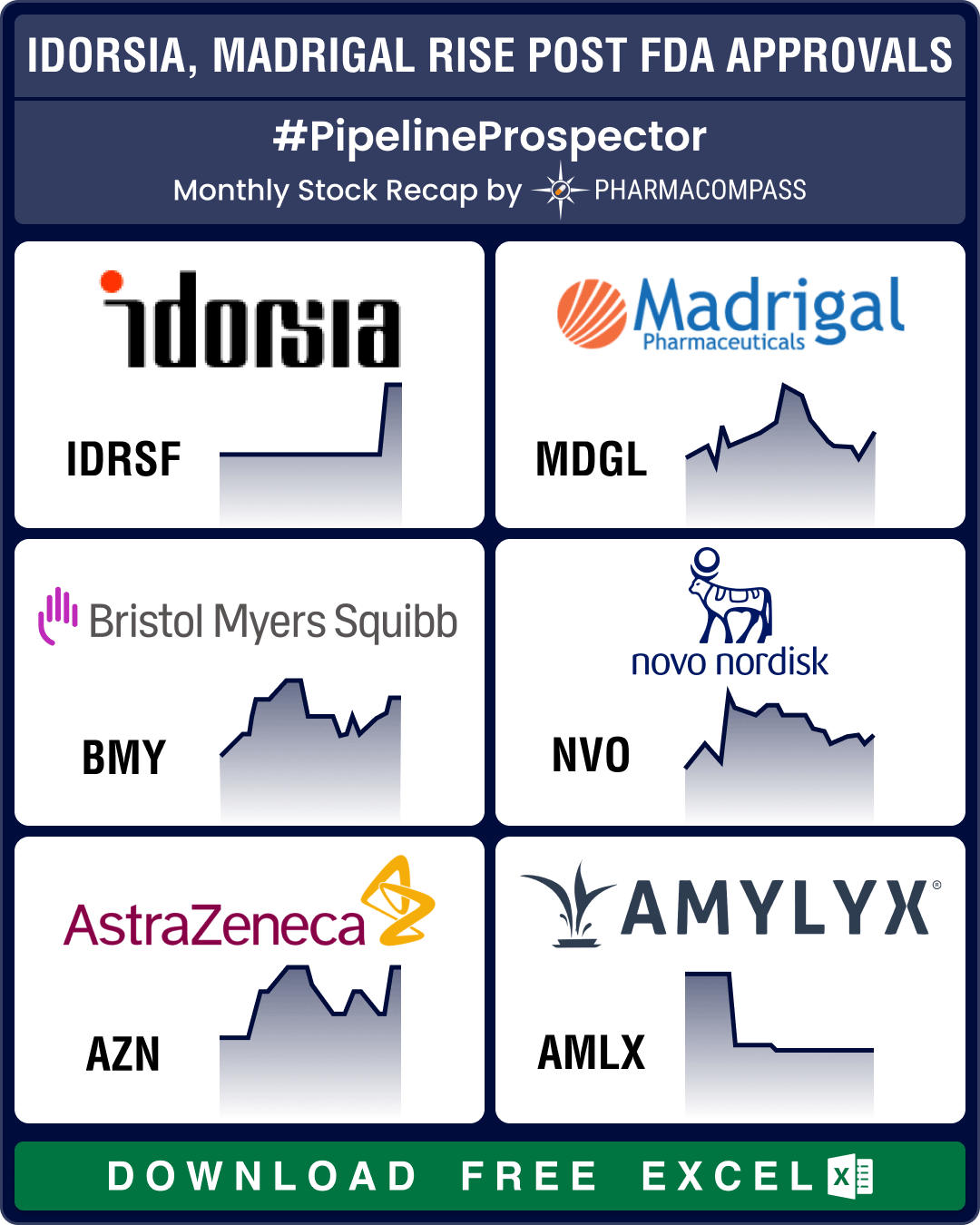
Pipeline Prospector March 2024: FDA approves pathbreaking NASH drug from Madrigal, two meds for PAH
March was clearly a month of drug approvals, as the US Food and
Drug Administration (FDA) went on a
Pipeline Prospector Jan 2024: Vertex’s non-opioid painkiller succeeds in trials; Sanofi buys Inhibrx for US$ 2.2 bn
The New Year got off to a stable start, with some good news
trickling in from clinical trials and B
Pipeline Prospector 2023 highlights: Obesity drugs perk up Lilly, Novo sales; ADCs spur dealmaking
The year 2023 was marked by volatility. The good news is that despite factors like inflation, intere


 Market Place
Market Place Sourcing Support
Sourcing Support