
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
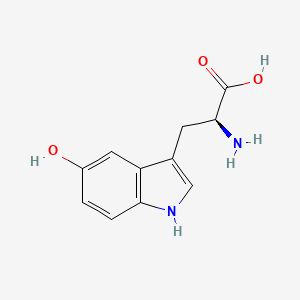

1. 5 Hydroxytryptophan
2. 5-htp
3. 5-hydroxy- Tryptophan
4. 5-hydroxytryptophan
5. Hydroxytryptophan
6. Oxitriptan
7. Oxytryptophan
8. Tryptophan, 5 Hydroxy
9. Tryptophan, 5-hydroxy-
1. L-5-hydroxytryptophan
2. Oxitriptan
3. 4350-09-8
4. 5-hydroxytryptophan
5. Cincofarm
6. Levothym
7. L-5-htp
8. Pretonine
9. Tryptophan, 5-hydroxy-
10. Quietim
11. 5-hydroxytryptophan L-form
12. Oxyfan
13. Hydroxytryptophan
14. (2s)-2-amino-3-(5-hydroxy-1h-indol-3-yl)propanoic Acid
15. Levotinine
16. Serotonyl
17. Triptene
18. Telesol
19. Tript-oh
20. (s)-2-amino-3-(5-hydroxy-1h-indol-3-yl)propanoic Acid
21. Oxitriptan [inn]
22. L-tryptophan, 5-hydroxy-
23. 5-htp
24. 5-hydroxyl-l-tryptophan
25. (s)-5-hydroxytryptophan
26. H-trp(5-oh)-oh
27. C1ljo185q9
28. Ro 3-5940 Hcl
29. Chebi:17780
30. Oxitriptan (inn)
31. Ncgc00091062-04
32. L-2-amino-3-(5-hydroxyindolyl)propionic Acid
33. Mfcd00064341
34. Dsstox_cid_5437
35. Dsstox_rid_77786
36. Dsstox_gsid_25437
37. Dl-oxitriptan
38. Tryptophan, 5-hydroxy-, L-
39. 5-hydroxy-l-tryptophane
40. 5-hydroxy Tryptophan
41. (s)-2-amino-3-(5-hydroxy-1h-indol-3-yl)propanoic Acid (5-hydroxytryptophan)
42. 5 Hydroxytryptophan
43. Cas-4350-09-8
44. 5-hydroxytryptophan, Dl-
45. 5-hydroxytryptophan L Form
46. Tryptophan, 5-hydroxy-, Dl
47. 5-22-14-00278 (beilstein Handbook Reference)
48. Oxitriptanum [inn-latin]
49. Oxitriptano [inn-spanish]
50. Oxitriptano
51. Oxitriptanum
52. Serotain
53. Tripten
54. L-oxitriptan
55. Ccris 4418
56. Levothym (tn)
57. Einecs 224-411-1
58. 5-hydroxytryptophane
59. Tryptophan, 5-htp
60. Brn 0088200
61. 5htp
62. Lopac-h-9772
63. Bmse000457
64. L-5-http
65. Oxitriptan [mart.]
66. 5-hydroxy-tryptophan
67. Oxitriptan [who-dd]
68. Unii-c1ljo185q9
69. Lopac0_000627
70. Schembl43243
71. Mls002153452
72. 5-hydroxytryptophan - 5-htp
73. (2s)-2-amino-3-(5-hydroxyindol-3-yl)propanoic Acid
74. 5-hydroxy L-tryptophan-[d4]
75. Chembl350221
76. Gtpl4671
77. 5-hydroxy-l-tryptophan, Powder
78. Hydroxytryptophan [inci]
79. Dtxsid1025437
80. Hydroxytryptophan [vandf]
81. Hms2231h15
82. Hms3261n16
83. Zinc895330
84. Hy-b1716
85. Tox21_111073
86. Tox21_201032
87. Tox21_500627
88. Bdbm50403163
89. Oxitriptan (5-hydroxy-l-tryptophan)
90. S4769
91. Akos004119863
92. Tox21_111073_1
93. Ccg-204715
94. Cs-w019879
95. Db02959
96. Lp00627
97. Sdccgsbi-0050608.p002
98. 5-hydroxy-l-tryptophan [usp-rs]
99. 5-hydroxytryptophan L-form [mi]
100. Ncgc00015526-01
101. Ncgc00091062-01
102. Ncgc00091062-02
103. Ncgc00091062-03
104. Ncgc00091062-05
105. Ncgc00091062-06
106. Ncgc00091062-07
107. Ncgc00091062-08
108. Ncgc00091062-09
109. Ncgc00091062-12
110. Ncgc00258585-01
111. Ncgc00261312-01
112. Ac-24420
113. As-12269
114. Smr001230815
115. Tryptophan Impurity D [ep Impurity]
116. Am20060629
117. Eu-0100627
118. H0531
119. 50h098
120. C00643
121. D07339
122. H 9772
123. L-5-hydroxytryptophan (l-5-htp; Oxitriptan)
124. A826297
125. Q238544
126. Sr-01000075584
127. N-acetyltryptophan Impurity D [ep Impurity]
128. Q-200544
129. Sr-01000075584-1
130. 2-amino-3-(5-hydroxy-1h-indol-3-yl)propionic Acid
131. S(+)-1-alpha-amino-5-hydroxyindole-3-propionic Acid
132. 5-hydroxy-l-tryptophan, 98% (calc. On Dried Substance)
133. Cdad59d9-d915-46dd-8029-48c808976b7c
134. 5-hydroxy-l-tryptophan, United States Pharmacopeia (usp) Reference Standard
135. 4pq
| Molecular Weight | 220.22 g/mol |
|---|---|
| Molecular Formula | C11H12N2O3 |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 220.08479225 g/mol |
| Monoisotopic Mass | 220.08479225 g/mol |
| Topological Polar Surface Area | 99.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 272 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
5-HTP has shown some usefulness in some conditions characterized, in part, by serotonin deficits, principally depression. It has also been shown to be useful in some with obesity, insomnia, fibromyalgia and chronic tension headache.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.4 (2001)
It has been long known that brain serotonin systems contribute to the modulation of food intake and satiety. An increase of intrasynaptic serotonin tends to reduce food consumption. Thus, one might consider that individuals taking 5-HTP might experience increase satiety and weight loss over a period of time. There are few studies on the effects of 5-HTP on obesity and they suggest an anorectic effect of 5-HTP.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 350 (2005)
There is some evidence that 5-HTP ... can improve postural equilibrium, dysarthria in patients with various inherited and acquired cerebellar ataxias, and particularly in those with lesions located precisely in the anterior lobe vermis. Improvements in coordination have been reported in patients with Friedreich"s ataxia; however, the effect is only partial and not clinically major.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 352 (2005)
Exptl Ther: Rats of the Dahl salt-sensitive (DS) and Dahl salt-resistant (DR) strains were placed on a 4% NaCl diet and blood pressures were monitored. Chronic subcutaneous infusion L-5-hydroxytryptophan (L-5-HTP, 12.6 mg/day) by osmotic minipumps significantly decreased the elevated systolic blood pressure of DS rats on a 4% NaCl diet. Blood pressures of DR rats were unaffected by treatment with L-5-HTP. Cardiac hypertrophy was associated with Dahl salt-induced hypertension. However, treatment with L-5-HTP failed to reduce the weight of the heart significantly. These results suggest that chronic administration of L-5-HTP was effective in reducing the elevated blood pressure in the DS model. The specific mechanisms by which L-5-HTP reduces the elevated blood pressure in DS rats is not clear and remains for further study.
PMID:1829234 Baron A et al; Pharmacology 42 (1): 15-22 (1991)
For more Therapeutic Uses (Complete) data for 5-HYDROXYTRYPTOPHAN (6 total), please visit the HSDB record page.
Other reported side effects, include nausea, diarrhea, loss of appetite, vomiting and difficult breathing. Neurological side effects, including dilation of the pupils, abnormally sensitive reflexes, loss of muscle coordination and blurring of vision, have been reported in those taking large doses of 5-HTP. Cardiac dysrhythmias have also been reported.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.5 (2001)
Eosinophilia and eosinophilia-myalgia syndrome (EMS) have been reported in those taking 5-HTP. The eosinophilia myalgia syndrome is similar to that caused by L-tryptophan and was linked to contaminants in the 5-HTP preparation, rather than 5-HTP itself. Changing the 5-HTP lot in one group of patients resolved the eosinophilia. A scleroderma-like skin condition has been reported in some taking a combination of 5-HTP and carbidopa.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.5 (2001)
5-HTP should be avoided by pregnant women and nursing mothers.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.5 (2001)
5-HTP should be avoided by those with ischemic heart disease (history of myocardial infarction, angina pectoris, documented silent ischemia), coronary artery spasm (e.g., Prinzmetal's angina), uncontrollable hypertension and any other significant cardiovascular disease.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.4 (2001)
For more Drug Warnings (Complete) data for 5-HYDROXYTRYPTOPHAN (8 total), please visit the HSDB record page.
For use as an antidepressant, appetite suppressant, and sleep aid.
The psychoactive action of 5-HTP is thought to be due to increased serotonin production in central nervous system tissue.
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX01 - Oxitriptan
The immediate precursor in the serotonin synthetic route, 5-hydroxytryptophan (5-HTP), labeled with 11C in the beta position, has become available for studies using positron emission tomography (PET) to examine serotonin formation in human brain. Normalized uptake and intracerebral utilization of tracer amounts of (beta-11C)5-HTP were studied twice in six healthy male volunteers, three of them before and after pharmacological pretreatments ... Pretreatments with benserazide, p-chlorophenylalanine (PCPA), and unlabeled 5-HTP all significantly increased uptake of (beta-11C)5-HTP. The utilization rates in both striatal and frontal cortex were higher than those in the surrounding brain, indicating that PET studies using (beta-11C)5-HTP as a ligand quantitate selective processes in the utilization of 5-HTP.
PMID:1292039 Reibring L et al; Psychiatry Res 45(4): 215-25 (1992)
The efficiency of absorption of 5HTP, as well as its decarboxylation product serotonin, is approximately 47% to 84%. Absorption of 5-HTP occurs by an active transport process. 5-HTP is transported by the portal circulation to the liver where approximately 25% of an administered dose is metabolized ... . 5-HTP that is not metabolized in the liver is transported by the general circulation to the various tissues of the body, including the brain. 5-HTP readily crosses the blood-brain barrier, and is converted to serotonin in brain cells.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.4 (2001)
5-Hydroxytryptophan is decarboxylated to serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase via a vitamin B6 dependent reaction in nervous tissue and in the liver.
5-HTP is transported by the portal circulation to the liver where approximately 25% of an administered dose is metabolized via vitamin B6-dependent L-aromatic amino acid decarboxylase to 5-hydroxytryptamine (5-HT) /serotonin/. 5-HT is subsequently metabolized to 5-hydroxyindole acetaldehyde which is rapidly metabolized to 5-hydroxyindole acetaldehyde which is rapidly metabolized to 5-hydroxyindoleacetic acid (5-HIAA).
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.4 (2001)
The possible analgesic effect of 5-HTP may be accounted for, in part, by its conversion to serotonin. 5-HTP has also been found to increase plasma beta-endorphin and platelet met-enkephalin levels, which may signify a reinforcing effect upon an endogenous analgesic effect.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.3 (2001)
The mechanism of the possible antidepressant activity of 5-HTP is accounted for by its conversion to the neurotransmitter serotonin which plays a central role in the affective state. Antidepressants may work by either binding to one or more of the family of serotonin 5-HT receptors (5-HT1 - 5-HT7) or by inhibiting the reuptake of serotonin. ...
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.3 (2001)
Global Sales Information

Market Place

ABOUT THIS PAGE
94
PharmaCompass offers a list of 5-Hydroxy Tryptophan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right 5-Hydroxy Tryptophan manufacturer or 5-Hydroxy Tryptophan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred 5-Hydroxy Tryptophan manufacturer or 5-Hydroxy Tryptophan supplier.
PharmaCompass also assists you with knowing the 5-Hydroxy Tryptophan API Price utilized in the formulation of products. 5-Hydroxy Tryptophan API Price is not always fixed or binding as the 5-Hydroxy Tryptophan Price is obtained through a variety of data sources. The 5-Hydroxy Tryptophan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 5-Hydroxy Tryptophan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 5-Hydroxy Tryptophan, including repackagers and relabelers. The FDA regulates 5-Hydroxy Tryptophan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 5-Hydroxy Tryptophan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A 5-Hydroxy Tryptophan supplier is an individual or a company that provides 5-Hydroxy Tryptophan active pharmaceutical ingredient (API) or 5-Hydroxy Tryptophan finished formulations upon request. The 5-Hydroxy Tryptophan suppliers may include 5-Hydroxy Tryptophan API manufacturers, exporters, distributors and traders.
click here to find a list of 5-Hydroxy Tryptophan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 5-Hydroxy Tryptophan DMF (Drug Master File) is a document detailing the whole manufacturing process of 5-Hydroxy Tryptophan active pharmaceutical ingredient (API) in detail. Different forms of 5-Hydroxy Tryptophan DMFs exist exist since differing nations have different regulations, such as 5-Hydroxy Tryptophan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 5-Hydroxy Tryptophan DMF submitted to regulatory agencies in the US is known as a USDMF. 5-Hydroxy Tryptophan USDMF includes data on 5-Hydroxy Tryptophan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 5-Hydroxy Tryptophan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 5-Hydroxy Tryptophan suppliers with USDMF on PharmaCompass.
5-Hydroxy Tryptophan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 5-Hydroxy Tryptophan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 5-Hydroxy Tryptophan GMP manufacturer or 5-Hydroxy Tryptophan GMP API supplier for your needs.
A 5-Hydroxy Tryptophan CoA (Certificate of Analysis) is a formal document that attests to 5-Hydroxy Tryptophan's compliance with 5-Hydroxy Tryptophan specifications and serves as a tool for batch-level quality control.
5-Hydroxy Tryptophan CoA mostly includes findings from lab analyses of a specific batch. For each 5-Hydroxy Tryptophan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
5-Hydroxy Tryptophan may be tested according to a variety of international standards, such as European Pharmacopoeia (5-Hydroxy Tryptophan EP), 5-Hydroxy Tryptophan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (5-Hydroxy Tryptophan USP).
