
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
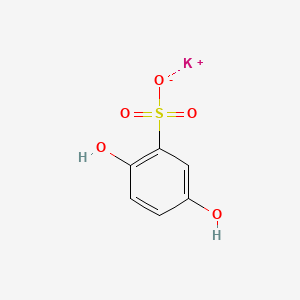

1. Potassium Dobesilate
1. 21799-87-1
2. Hydroquinonesulfonic Acid Potassium Salt
3. Potassium Dobesilate
4. 2,5-dihydroxybenzenesulfonic Acid Potassium Salt
5. Benzenesulfonic Acid, 2,5-dihydroxy-, Monopotassium Salt
6. Potassium Hydroquinonesulfonate
7. Potassium;2,5-dihydroxybenzenesulfonate
8. 249e3f00ep
9. Benzenesulfonic Acid, 2,5-dihydroxy-, Potassium Salt (1:1)
10. Smr000875261
11. Mfcd00007475
12. Khqs
13. Potassium,2,5-dihydroxybenzenesulfonate
14. Potassium 2,5-dihydroxybenzenesulphonate
15. Hydroquinone Sulfonic Acid,potassium Salt
16. Dsstox_cid_24410
17. Dsstox_rid_80209
18. Dsstox_gsid_44410
19. Schembl78223
20. Mls001333259
21. Mls001333260
22. Unii-249e3f00ep
23. Chembl1707052
24. Dtxsid8044410
25. Amy8945
26. Hms2234i14
27. Waa79987
28. Einecs 244-584-7
29. Tox21_302096
30. Akos015889677
31. Ncgc00255728-01
32. As-64019
33. Cas-21799-87-1
34. Db-045700
35. Potassium 2,5-dihydroxybenzene-1-sulfonate
36. Cs-0157160
37. Ft-0625555
38. H0593
39. E76054
40. A815671
41. Hydroquinone Sulfonic Acid, Potassium Salt
42. W-107519
43. 2,5-dihydroxybenzenesulfonic Acid, Monopotassium Salt
44. Q27253834
45. Hydroquinonesulfonic Acid Potassium Salt, Technical Grade
| Molecular Weight | 228.27 g/mol |
|---|---|
| Molecular Formula | C6H5KO5S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 227.94947591 g/mol |
| Monoisotopic Mass | 227.94947591 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?