Medical Breakthroughs in 2024: Alzheimer’s, schizophrenia, COPD, MASH see pathbreaking treatments
This year has seen pivotal advancements in medical innovation. The US Food and Drug Administration (
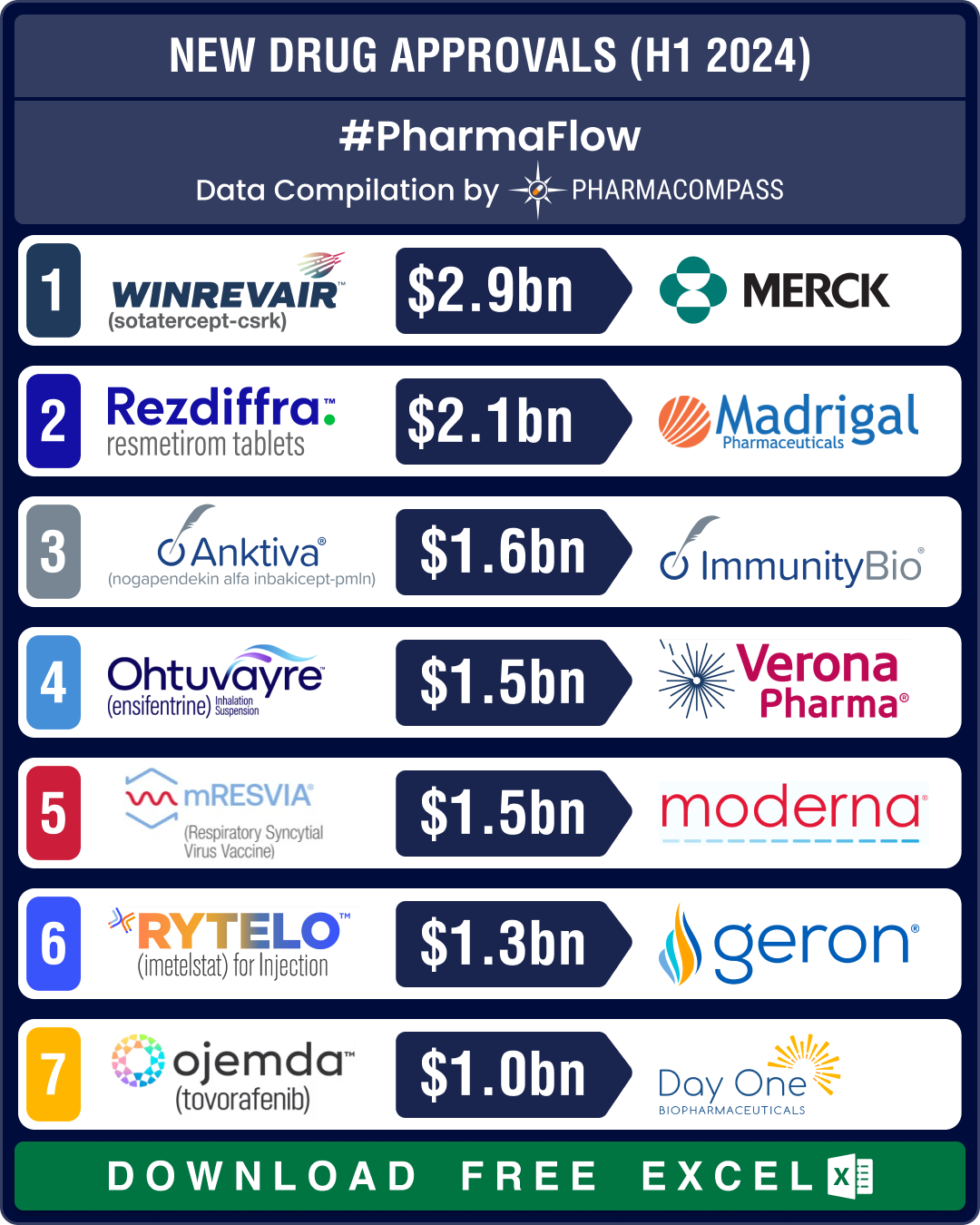
FDA approvals slump 19% in H1 2024; NASH, COPD, PAH get new treatment options
The first half of 2024 saw a significant slowdown in approvals of new drugs and biologics by the US
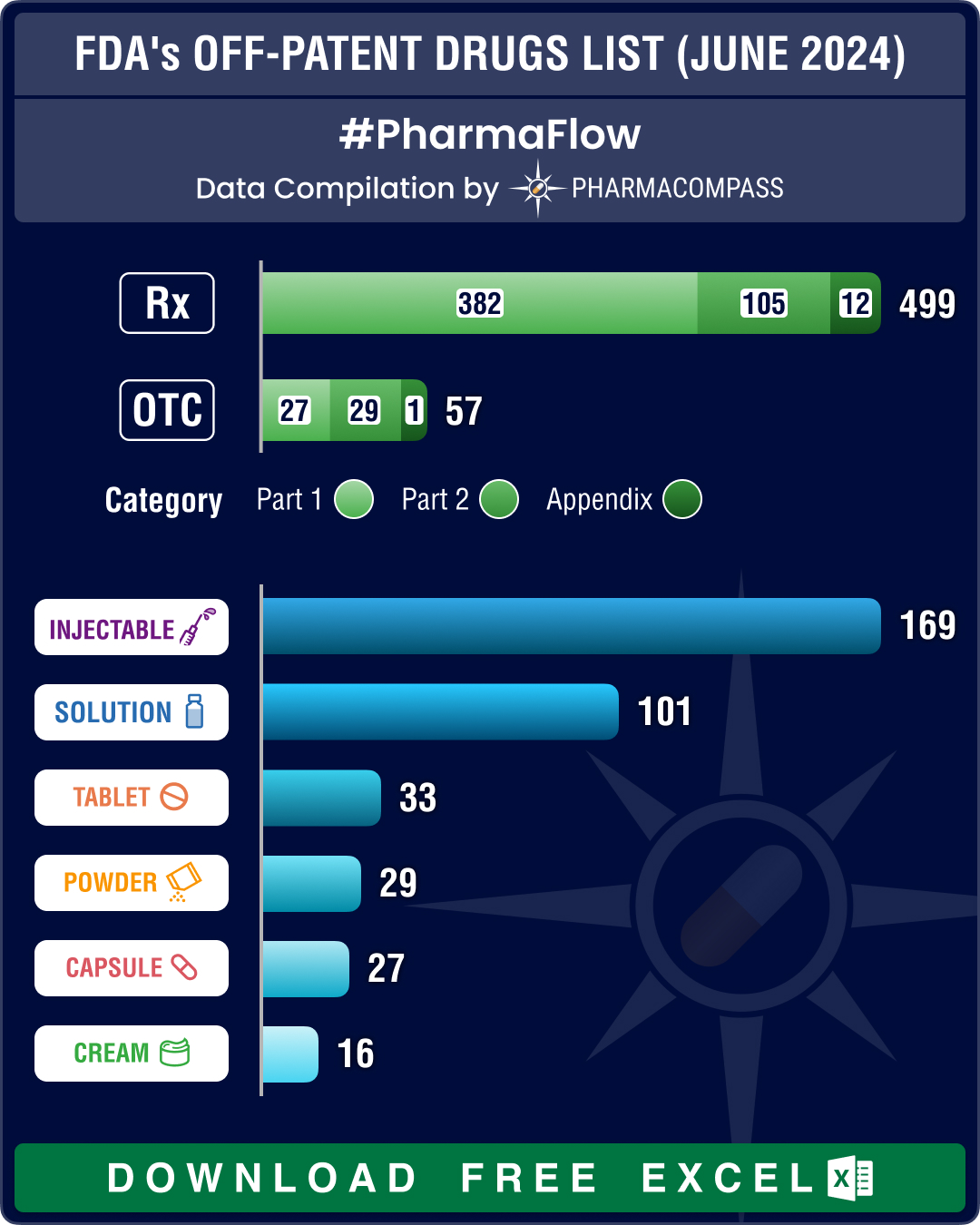
FDA’s June 2024 list of off-patent, off-exclusivity drugs sees rise in cancer, HIV treatments
This week PharmaCompass brings to you key highlights of the US Food and Drug Administration’s
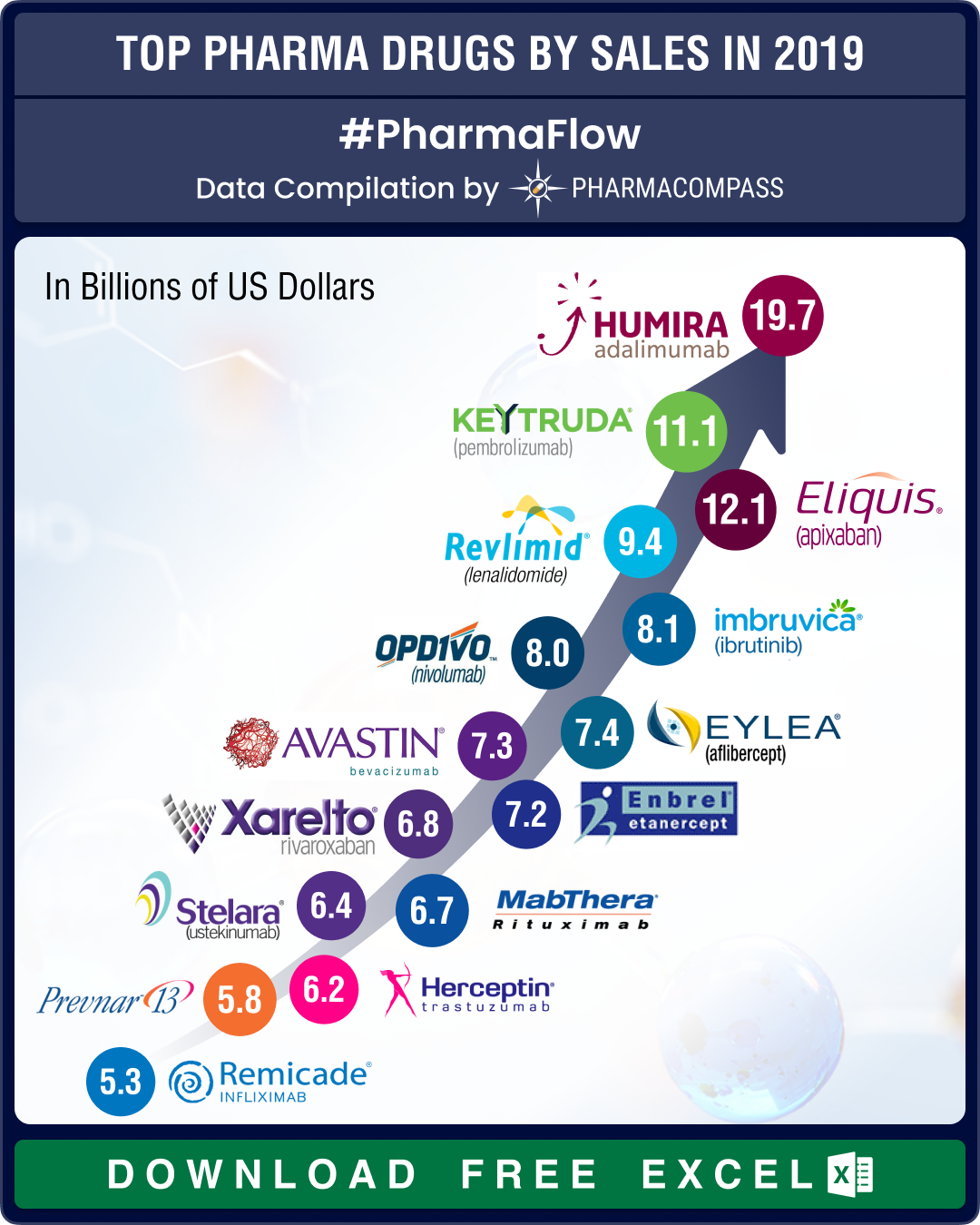
Top drugs and pharmaceutical companies of 2019 by revenues
Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-
Compliance to determine the success of Mylan’s merger with Pfizer’s Upjohn
This week, Mylan and Pfizer announced the merger of Upjohn, Pfizer’s off-patent branded and ge
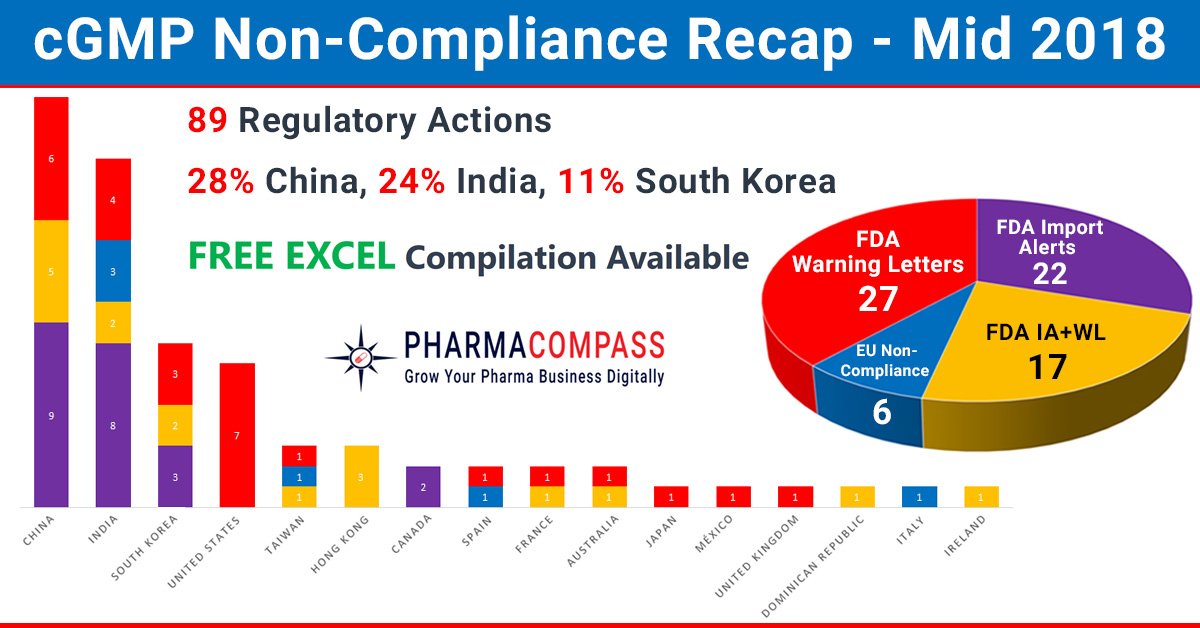
Mid 2018 – Recap of Warning Letters, Import Alerts and Non-Compliances
In our mid-2018 compliance review, we look at inspection challenges
faced by companies across the w
Compliance Recap: Data-integrity threatens US $4.3 billion Fresenius-Akorn deal
In April last year, German drugmaker Fresenius Kabi had agreed to acquire Akorn, a manufacturer and
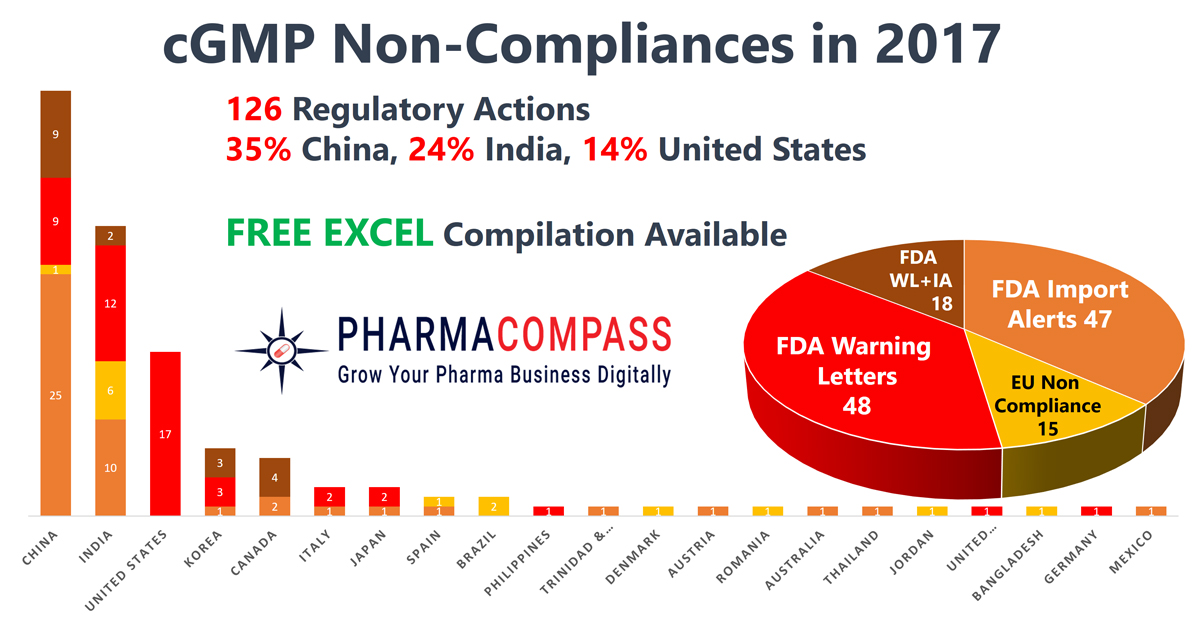
2017 – Recap of Warning Letters, Import Alerts and Non-Compliances
Data
integrity continued to be a hot topic in the pharmaceutical industry through
2017. According to
Teva’s rating cut to junk; Two Lupin facilities receive FDA warning letters
This week in Phispers, we look at the contrasting 2017 forecasts of Mylan and Teva, resulting from t


 Market Place
Market Place Sourcing Support
Sourcing Support