Molecular glue degraders: Lilly, AbbVie sign billion-dollar deals; BMS leads with three late-stage drugs
This week, we delve into molecular glue degraders (MGDs), one of the most promising frontiers in dru
Top first-in-class drug candidates of 2025: Ionis’ donidalorsen, Sanofi’s fitusiran, Cytokinetics’ aficamten await FDA approval
First‑in‑class drugs are therapies with entirely new approaches that improve patient out
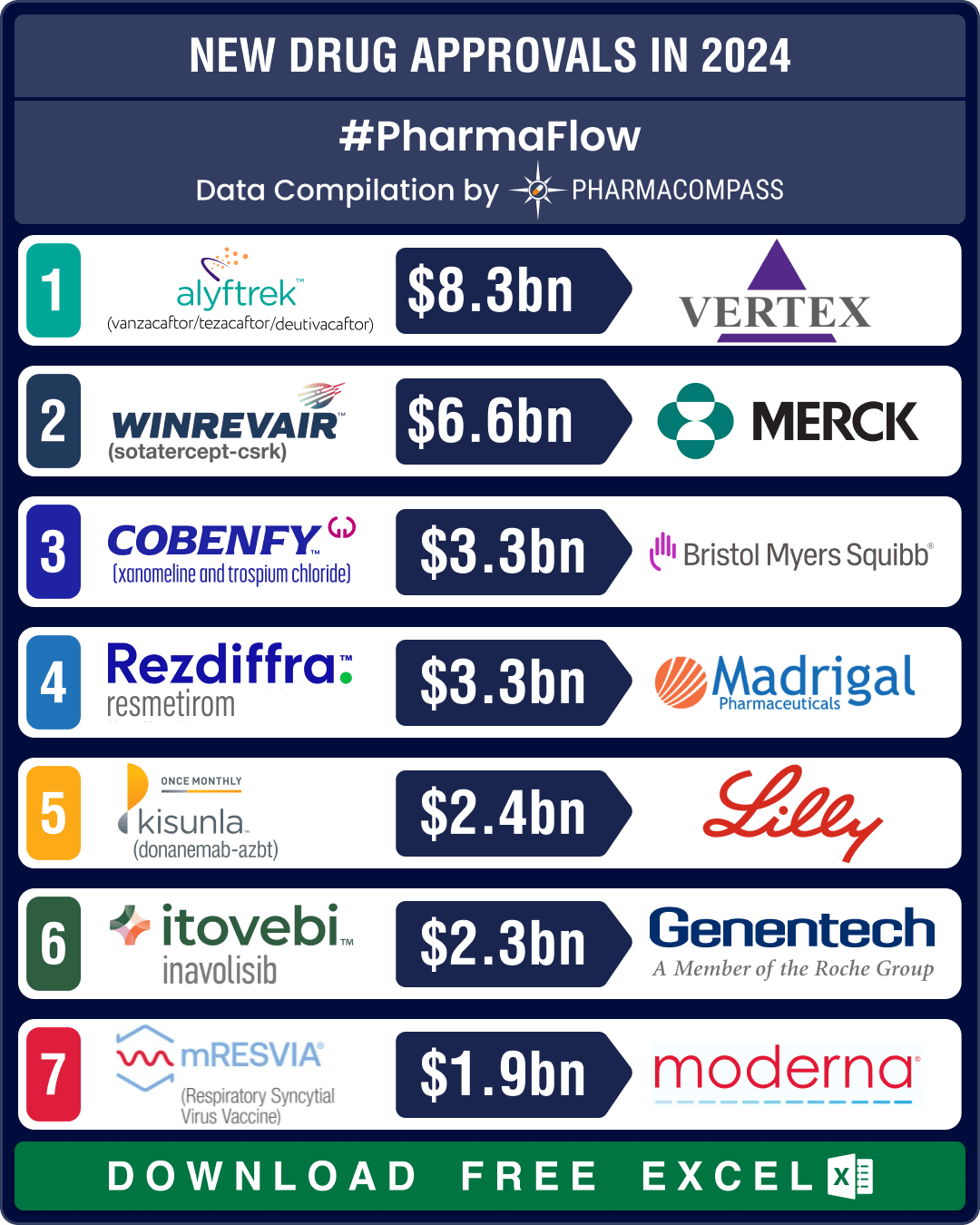
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
FDA’s landmark approvals of BMS’ schizo med, Madrigal’s MASH drug, US$ 16.5 bn Catalent buyout make it to top 10 news of 2024
The year 2024 was marked by some landmark drug approvals in the areas of schizophrenia, metabolic dy
Chinese FDA-registered generic facilities gain steam, India maintains lead with 396 facilities
Every year, the US Food and Drug Administration (FDA) publishes the user fee amounts it will collect
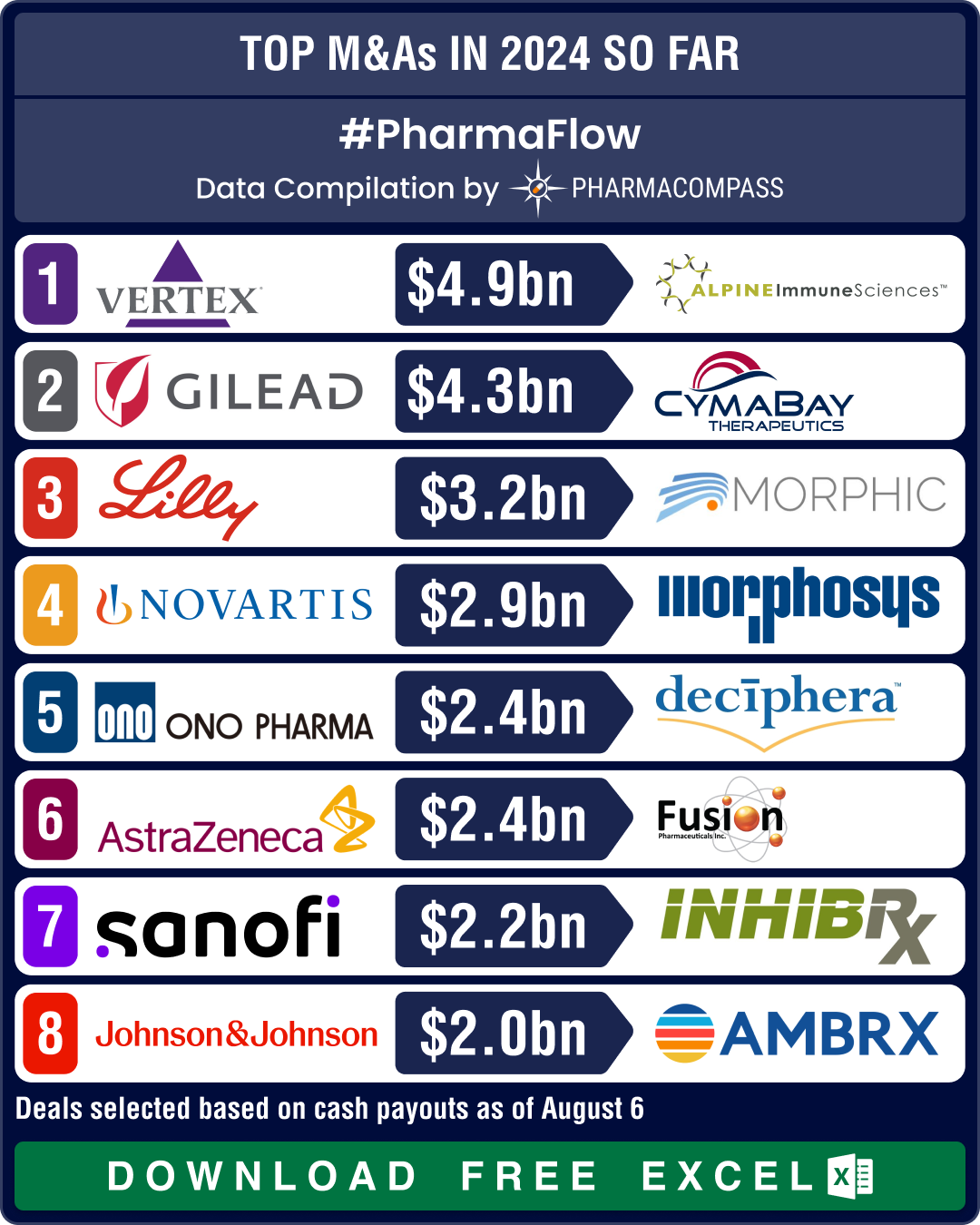
Novartis, GSK, Sanofi, BMS shell out over US$ 10 bn in dealmaking, as mid-size deals take centerstage in 2024
The world of pharmaceuticals and biotechnology continued to evolve
this year with strategic allianc
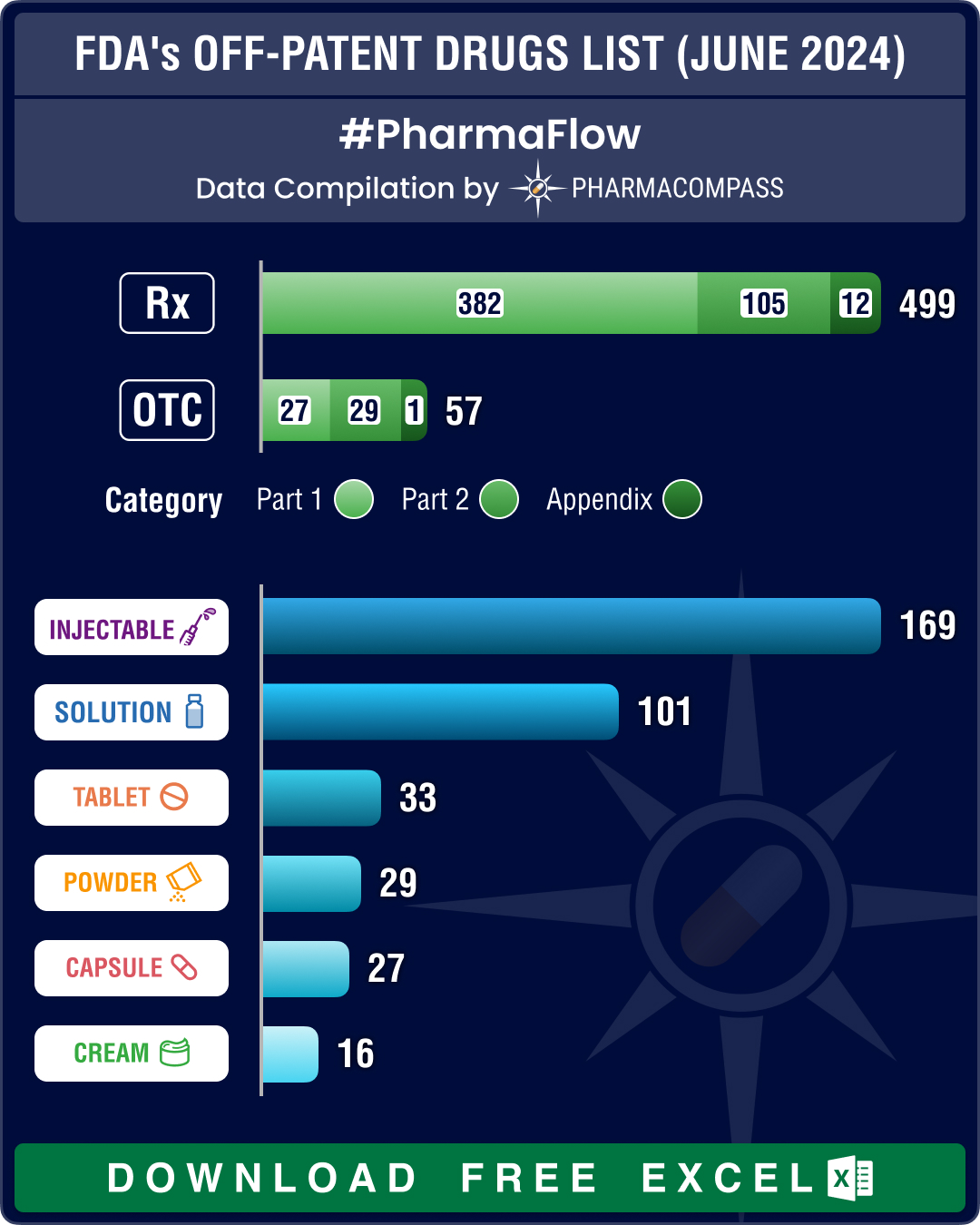
FDA’s June 2024 list of off-patent, off-exclusivity drugs sees rise in cancer, HIV treatments
This week PharmaCompass brings to you key highlights of the US Food and Drug Administration’s
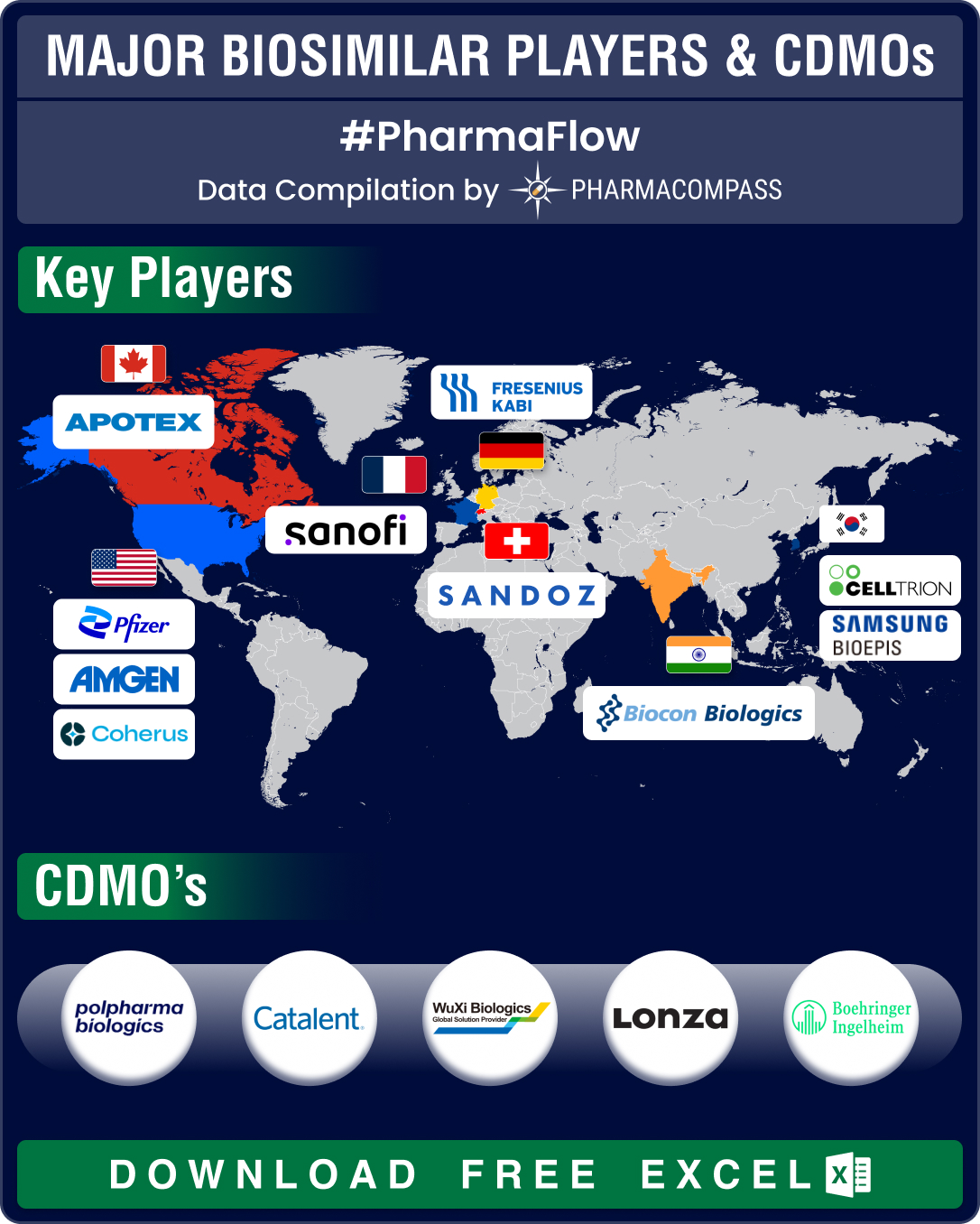
FDA approves record eight biosimilars in H1 2024; okays first interchangeable biosimilars for Eylea
Biologics, or complex drugs that are derived from living organisms, have revolutionized treatment of
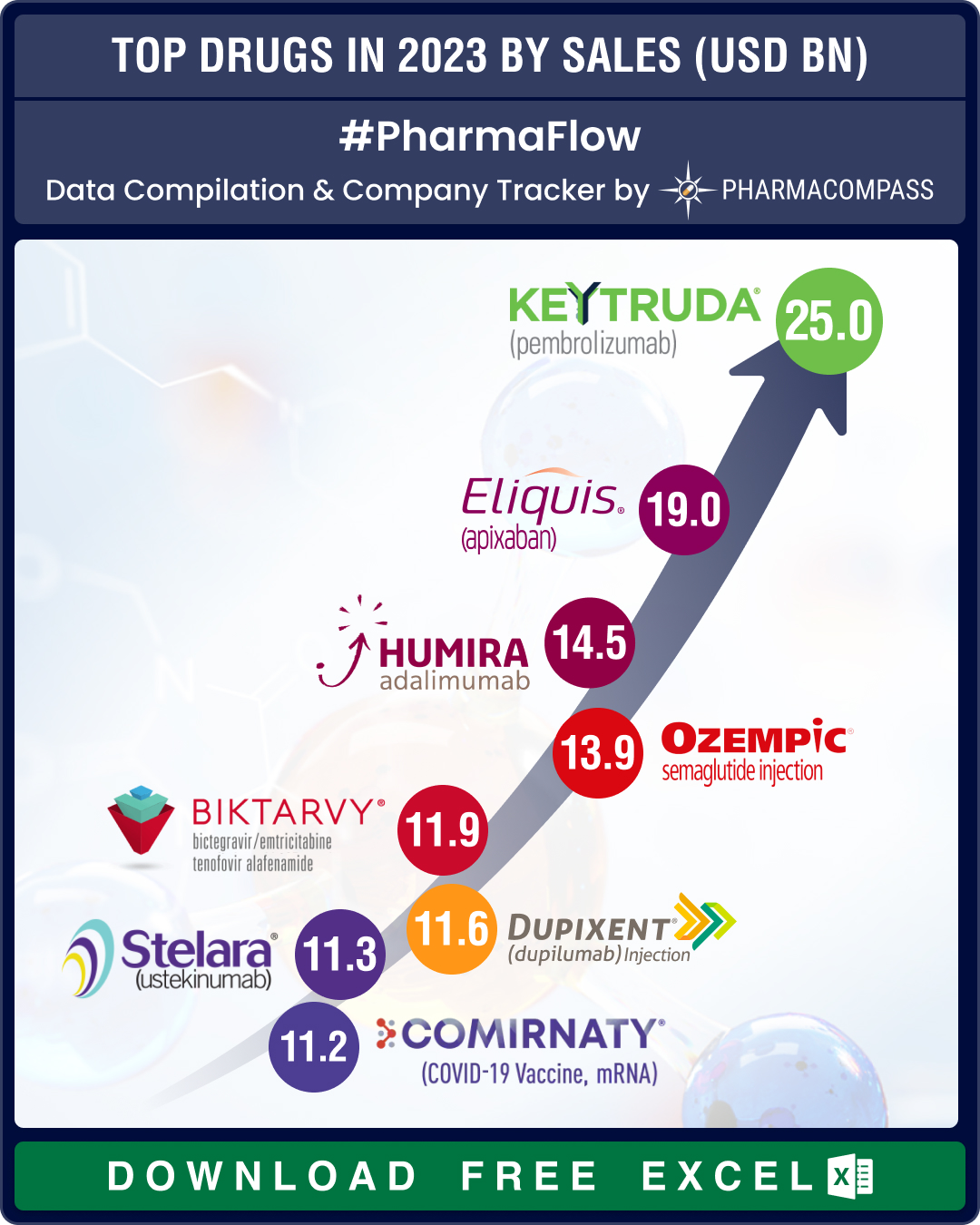
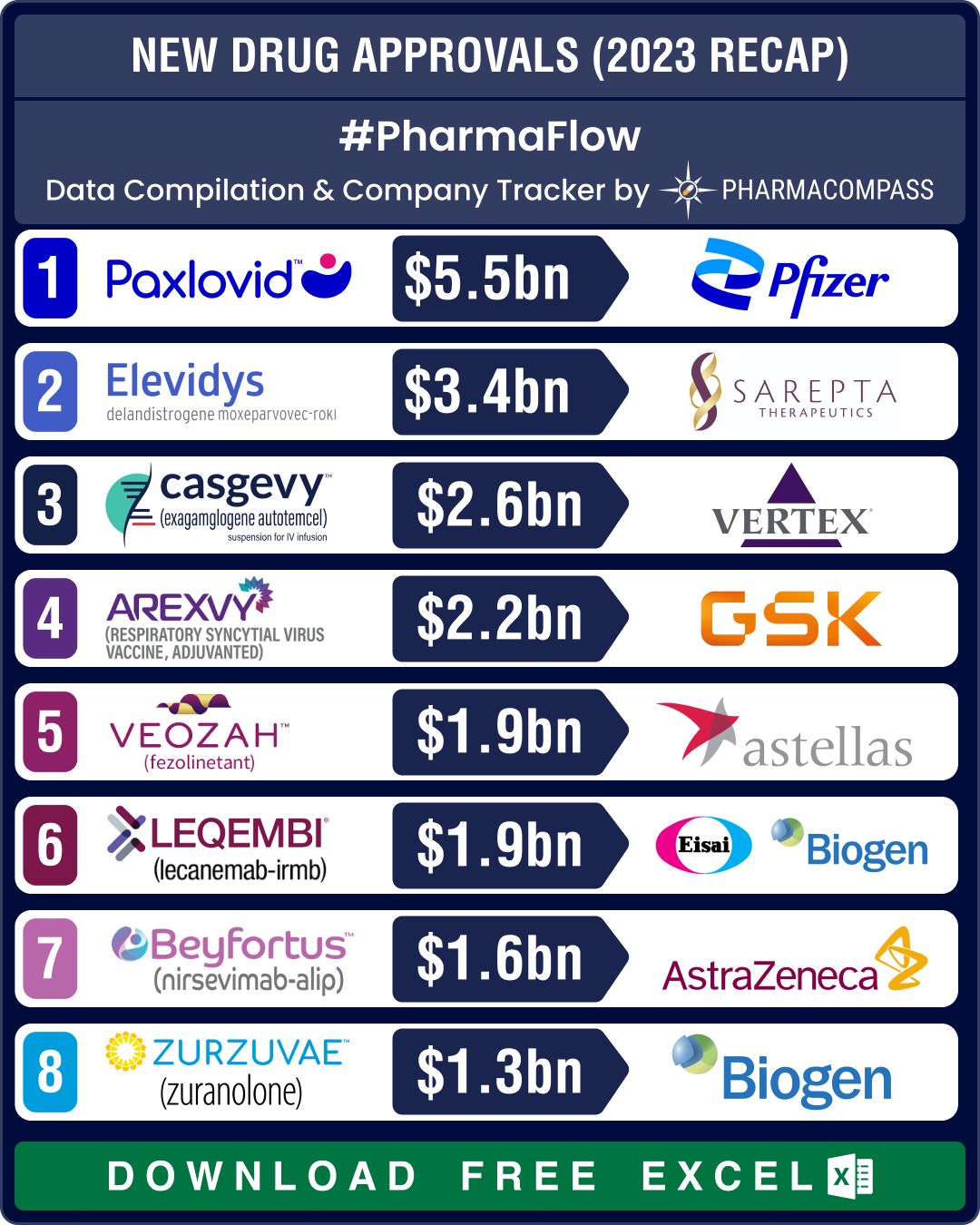
Top Pharma Companies & Drugs in 2023: Merck’s Keytruda emerges as top-selling drug; Novo, Lilly sales skyrocket
The pharma industry clearly recalibrated itself in 2023, turning its focus away from Covid and onto


 Market Place
Market Place Sourcing Support
Sourcing Support