By PharmaCompass
2022-01-06
Impressions: 2740
Nearly every
year, drugmakers ring in the new year with drug price increases in the US. This
year too, prices of over 450 prescription
medicines increased by an average of around 5 percent at the start of January.
This, when high drug prices have been one of the biggest political issues in
the US over the last few years.
PharmaCompass decided to usher in 2022 with a review of the US Medicare Part D Prescription Drug data recently released by the Centers for Medicare and Medicaid Services (CMS) for calendar year 2019. Using the available data, we have developed our own dashboard to show recent trends in consumption of prescription drugs. With this analysis, we hope our readers will get a better understanding of the world’s largest market for pharmaceuticals, as also a fix on where it may be headed.
View US Medicare Part D 2019 Drug Spending (Free Excel Available)
Rising healthcare, drug spends in US
Over the last several years, we have repeatedly heard political leaders in the US complain about high drug prices. Yet, drug prices and healthcare spends have risen unabated.
America’s National Health Expenditure Accounts (NHEA) includes annual expenditures on healthcare goods and services, public health activities, the net cost of health insurance, and investment related to healthcare. In 2019, America’s national health expenditure (NHE) grew by 4.6 percent to US$ 3.8 trillion, accounting for 17.7 percent of the gross domestic product (GDP). During the year, prescription drug spend increased by 5.7 percent to US$ 369.7 billion. In comparison, Medicare spend grew 6.7 percent to US$ 799.4 billion.
President Joe Biden recently stressed on the need to cap the prices of essential drugs, and said that the average American pays the highest prices for prescription drugs anywhere in the world. Americans pay 10 times as much as other countries for life-saving insulin — the top selling prescription drug covered by the Part D program.
Pharma companies, on the other hand, have vehemently argued against any price cuts in the US, saying price cuts would hinder drug research and development for all diseases.
View US Medicare Part D 2019 Drug Spending (Free Excel Available)
Patented drugs account for 80.3 percent of total Part D spend
Medicare is the US federal government’s program that provides health insurance to most people who are 65 years or older. Medicare’s Part D plan provides outpatient drug coverage through private insurance companies that have contracts with the federal government. Eligible people have to choose and enroll in a private prescription drug plan for Part D coverage. Medicare Part B, on the other hand, covers a wide variety of medically necessary outpatient services and some preventative services.
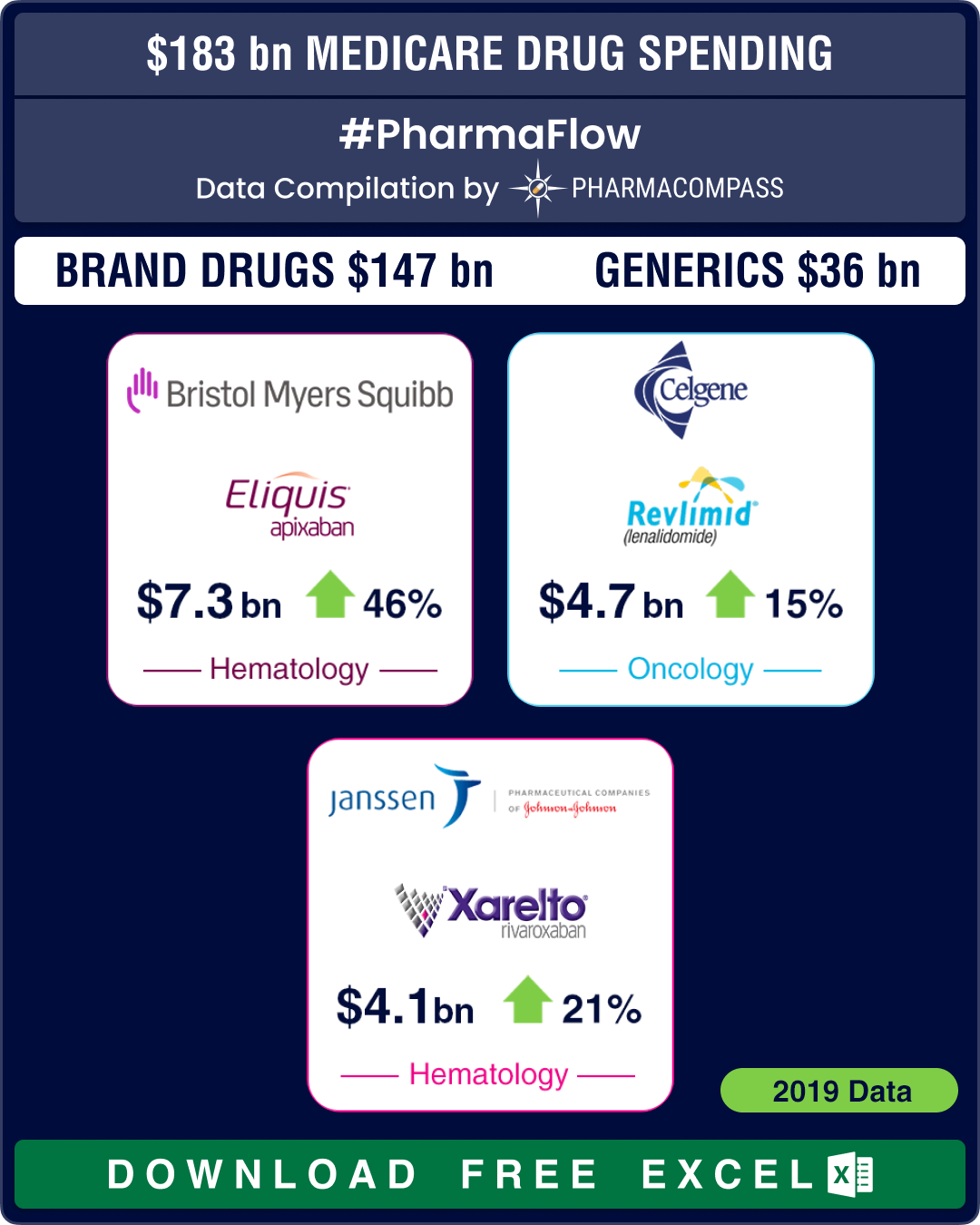
Prescription drug coverage under Part D reached US$ 183 billion in 2019 — a growth of around 9 percent over 2018, when spending was US$ 168 billion. Spending on patented drugs in 2019 accounted for around US$ 147 billion or 80.3 percent of the total spend for the year. Generic drugs made up for the remaining 19.7 percent (approximately US$ 36 billion). In 2018, generic drugs worth US$ 35.8 billion were sold under Part D, accounting for 21 percent of the total spend under the program.
View US Medicare Part D 2019 Drug Spending (Free Excel Available)
Eliquis ranks highest on Medicare’s brand drug spend
Under Part D, endocrinology and oncology were the two therapeutic areas that generated maximum revenue for pharma companies, driving home sales of over US$ 31.8 billion and US$ 23.5 billion, respectively. Neurology drugs generated sales of around US$ 22.9 billion.
Among branded drugs, Bristol Myers Squibb’s anticoagulant Eliquis (apixaban) was the most selling drug in 2019 under Part D, notching up about US$ 7.3 billion in sales — a rise of US$ 2.3 billion or 46 percent over 2018.
Celgene’s cancer drug Revlimid (lenalidomide) roped in US$ 4.7 billion (up by 14.6 percent), while another anticoagulant drug Xarelto (rivaroxaban) by Janssen Pharma — a unit of Johnson & Johnson — fetched US$ 4.1 billion (up 20.6 percent) in sales through Part D. AbbVie’s anti-rheumatic drug Humira and Sanofi’s diabetes drug Lantus saw sales of around US$ 3.7 billion each under the program.
Amongst generics, the largest selling drug under Part D (by dosage units) was metformin (diabetes), followed by gabapentin (seizure), PEG3350 with electrolyte (gastroenterology), metoprolol (hypertension) and atorvastatin (cholesterol). In 2019, the overall dosage units sold also jumped higher by 2.25 billion units to 111.35 billion.
The sales ranking of Part D does bare some similarities with the global ranking of highest selling drugs. In 2020, Humira had retained its position as the highest selling drug in the world, generating sales of US$ 20.4 billion. Both Eliquis and Revlimid had retained their ranking as the third and fourth most selling drugs, bringing home US$ 14.1 billion and US$ 12.1 billion in global sales in 2020.
View US Medicare Part D 2019 Drug Spending (Free Excel Available)
Medicare’s inability to negotiate prices costs American taxpayers billions of dollars
Over the years, drug companies have used Medicare’s inability to negotiate prices under Part D to increase the prices of their drugs significantly and rip off huge profits, a three-year-long US House Oversight Committee investigation has revealed.
US taxpayers could have saved over US$ 25 billion in five years if the prices of just seven drugs — Humira, Imbruvica, Sensipar, Enbrel, Lantus, NovoLog and Lyrica — were negotiated by Medicare. Another US$ 16.7 billion could have been saved between 2011 and 2017 on insulin products manufactured by Eli Lilly, Novo Nordisk and Sanofi, which control 90 percent of the insulin market in the US, the committee’s report revealed.
Elsewhere in the world, the same drugmakers are bending over backwards to get into medical insurance programs. For instance, China reported that several international pharma firms, many of them headquartered in the US, slashed the prices of their drugs by up to 94 percent to get into the country’s national medical insurance coverage.
In the US — which accounted for around 46 percent of the global share of drugs in 2020 — senior citizens may have to pay more for medicines as the government announced a large hike in Medicare premiums for 2022 if an expensive Alzheimer’s drug, Aduhelm, is included in the list.
In order to ensure inclusion in Medicare, Biogen slashed the price of Aduhelm by half — from US$ 56,000 to US$ 28,200 — just weeks before a crucial meeting called by the CMS. Clearly, this has set a precedent in an industry which is known for rampant price hikes and rarely for any price cuts. This could also be put forth as an example of what Medicare could achieve if it receives negotiation rights.
View US Medicare Part D 2019 Drug Spending (Free Excel Available)
Our view
President Biden's Build Back Better legislation, which the House passed last month, is up for vote in the Senate. The legislation contains provisions that would allow Medicare to negotiate the prices of some expensive drugs, penalize drugmakers who raise prices faster than inflation and cap out-of-pocket costs for insulin at US$ 35 per month. However, chances of the bill being passed in its present form are slim.
Even if the Senate passes the bill, Medicare would be able to negotiate the prices of only 10 prescription drugs and insulin products in 2025. The number would increase over the years, reaching 100 in six years, and hence forth grow by 20 drugs a year.
It seems like 2022 won’t be the last year when January 1 will be braced with price hikes in the US by drugmakers. Looks like they will continue to make hay while the sun shines.
View US Medicare Part D 2019 Drug Spending (Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Medicare Part D 2019 Drug Spending by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








