In the intricate world of molecular biology, oligonucleotides stand out as versatile, powerful molecules. Oligonucleotides are essentially short, single strands of DNA or RNA that modulate gene expression. There are various oligonucleotide therapy (ONT) agents (such as antisense, deoxyribozymes, siRNA and CRISPR/Cas) that offer promising therapeutic tools.A variation of gene therapy, oligonucleotide gene therapies (OGTs) are manufactured using synthetic oligonucleotides. These therapies are designed to enter cells. ONTs act like tools that can fine-tune the behavior of certain genetic instructions, and are therefore often designed to treat rare and genetic diseases and cancers. Sometimes, they may be delivered into cells through lipid nanoparticles or adeno-associated viruses (AAV), halting the translation of a specific protein. Oligonucleotides have also revolutionized vaccine development through the creation of nucleic acid vaccines, such as mRNA vaccines.The first oligonucleotide
drug, known as fomivirsen, was approved by the
US Food and Drug Administration (FDA) back in 1998. It was developed by Ionis Pharmaceuticals (then known as Isis Pharmaceuticals), and was approved to treat a rare eye disease. However, ONT approvals have picked up since 2016. Currently, there are 20 oligonucleotide drugs approved by the FDA and the European Medicines
Agency (EMA) and a majority of them treat orphan and rare
diseases.In 2023, FDA approved four ONTs — AstraZeneca-Ionis’ Wainua (eplontersen), Novo Nordisk owned Dicerna Pharmaceuticals’ Rivfloza (nedosiran), Biogen-Ionis’ Qalsody (tofersen) and Iveric Bio’s Izervay (avacincaptad pegol).In 2021, the global ONT market size was estimated to be US$ 18.2 billion. It is expected to increase to US$ 51.4 billion by 2029, growing at a compounded annual rate (CAGR) of 13.85 percent. Similarly, the market for oligonucleotides synthesis (or the process of producing oligonucleotides) was estimated at US$ 7.7 billion globally in 2022
and is expected to grow 11.8 percent CAGR to reach US$ 16.4
billion by 2030. Some of the bigger players in oligonucleotides synthesis are Danaher Corporation, Thermo Fisher Scientific, Merck KGaA, Eurofins, Agilent, Bio-Synthesis, EUROAPI, Eurogentec, STA Pharma, and Bachem.View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available)ONTs address neuro disorders; four ONTs bring in US$ 1.24 bn for AlnylamONTs are widely applied to treat
neurodegenerative diseases, such as Duchenne muscular dystrophy (DMD). Multiple ONTs have been approved in Europe and the US for
DMD, such as Exondys 51 (eteplirsen), Vyondys 53 (golodirsen), and Amondys 45
(casimersen) from Sarepta and NS Pharma’s Viltepso (viltolarsen). Last year, Ionis bagged two approvals in the
space. FDA
approved its Biogen-partnered
therapy Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis
(ALS). The agency
also approved AstraZeneca and Ionis’ drug Wainua (eplontersen), rendering it as the
first self-administered treatment for a rare nerve damage
disease, known as hereditary transthyretin amyloidosis (ATTR-PN). Analysts
estimate global peak sales for Wainua to come in at US$ 750
million for ATTR-PN alone. The drug is also being
tested for transthyretin-mediated amyloid cardiomyopathy (ATTR-CM), a rare
heart muscle disorder.Alnylam
Pharmaceuticals has also successfully brought four ONTs to market in recent years — Onpattro (patisiran) and
Amvuttra (vutrisiran) for rare
nerve diseases, and Givlaari (givosiran) and Oxlumo (lumasiran). Givlaari has been approved to treat acute hepatic porphyria (a liver enzyme deficiency) while Oxlumo treats primary hyperoxaluria (a disorder characterized by increased urinary oxalate excretion). The four ONTs brought in US$ 1.24
billion for Alnylam in 2023.View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available) Novartis
buys rights to siRNA therapy, GSK bets big on ONT pipeline through dealsAfter Alnylam discovered
inclisiran (Leqvio), Novartis acquired
global rights to the therapy in a US$ 9.7
billion deal. Leqvio was the first
FDA-approved small interfering RNA (siRNA) therapy
for LDL-C (bad cholesterol) reduction. It brought in US$ 355
million for Novartis in 2023.GSK has increasingly
been investing
in its ONT pipeline. The British
pharma has promised over US$ 5 billion in upfront and milestone payments in
multiple deals. In February, GSK exercised
its option to license Elsie Biotechnologies’ discovery platform to find
and develop novel ONTs. GSK has also entered a discovery collaboration with Wave Life Sciences.Last July, Japanese drugmaker Astellas Pharma completed its
approximately US$ 5.9 billion buyout of New
Jersey-headquartered Iveric Bio. Iveric focuses on retinal diseases and the deal gives Astellas drug candidates to treat about 160 million people with eye ailments. Subsequently, in August, Iveric’s Izervay (avacincaptad pegol) was approved by the FDA as a new
treatment for geographic atrophy (GA) secondary to age-related macular
degeneration (AMD).After Novo Nordisk acquired RNAi technology company Dicerna Pharmaceuticals for US$ 3.3 billion in 2021, the latter’s once-monthly RNAi therapy Rivfloza (nedosiran) saw FDA approval last October. Rivfloza was
okayed for children nine years and older to treat a rare genetic condition that affects the kidneys,
known as primary hyperoxaluria type 1 (PH1).View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available) Ionis tees up NDAs for rare
disease treatments after two late-stage trial winsThe industry is looking for cures to a wide spectrum of diseases like cancer (such as melanoma, pancreatic, liver, glioblastoma, breast and ovarian cancer), cystic fibrosis, Alzheimer’s disease, Parkinson’s disease, hepatitis B, asthma, Rett syndrome, non-alcoholic steatohepatitis, and IgA nephropathy through its research on ONTs.In January, Ionis announced late-stage results wherein its
RNA-targeted hereditary angioedema (HAE) candidate, donidalorsen, significantly
reduced the rate of HAE attacks in patients
treated every four weeks and patients treated every eight weeks. The
California-based biotech is readying a new drug application (NDA) to submit to the FDA. HAE is a rare and life-threatening genetic disease that causes unpredictable and frequent severe swelling of the skin, gastrointestinal (GI) tract, upper respiratory system, face and throat. This year, Ionis’ olezarsen was granted fast track designation by
the FDA for the rare genetic disease familial chylomicronemia syndrome (FCS).
Last September, olezarsen had met its primary endpoint of reducing abnormally high
levels of triglycerides in a late-stage trial in patients with the metabolic disorder. Currently, there are no
FDA-approved treatments for this condition. If okayed, olezarsen is likely to
bring in US $849 million in sales for Ionis by 2032.In March, FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 12 to two in favor of the clinical
benefit/risk profile of imetelstat for the treatment of transfusion-dependent (TD) anemia in certain adult patients with myelodysplastic syndromes. The agency assigned a Prescription Drug User Fee Act (PDUFA) target action date of June 16, 2024, for Geron’s NDA for imetelstat. It is among the most anticipated drug launches this year.View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available) Our viewDeveloping ONTs is a field fraught with challenges, such as toxicity and drug delivery. There are safety concerns as well as concerns around delivering the therapy. However, technological breakthroughs and collaborations between pharma firms and contract research organizations that focus on drug delivery are continuously working towards addressing these challenges. All in all, we foresee exciting times ahead for ONTs.
Impressions: 2765
The year 2023 has
seen considerable job cuts by biopharmaceutical companies. While layoffs have
become commonplace since 2022, the exercise touched new heights this year. The reasons behind these job cuts have ranged from restructuring, drop in Covid sales, fall in quarterly revenues to shift in strategy and mergers and acquisitions.In 2022, over 100
biopharma companies had announced workforce reductions. That number has doubled in 2023. And the year has not ended as yet.In Q1 2023, the
number of companies that had announced layoffs surpassed 50 and included
companies like Biogen and Thermo Fisher. Between mid-April and November, this
figure rose even further, with approximately 140 pharmaceutical companies
disclosing workforce reductions.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Biogen, Amgen, layoff staff post mergers;
low Q2 sales trigger job cuts at BMSIn
our Q1 2023 update, we had mentioned that both Novartis and Biogen plan to cut jobs worldwide
to save US$ 1 billion in costs. Starting November, Novartis will retrench over 100 employees at its US headquarters in East Hanover (New Jersey). Similarly, Biogen
announced plans to layoff around 113 employees from Reata Pharmaceuticals’ Plano, Texas site in the US. Biogen had acquired Reata for US$ 7.3 billion in July this year, and the job cuts were announced promptly after the acquisition.Another company
that announced post-merger retrenchments is Amgen, which had acquired Horizon Therapeutics in December 2022 for US$ 27.8 billion. Amgen has been aggressively cutting
costs. Earlier this year, it had laid off 750 employees due to intensifying pressure on drug
prices and inflation. And now, it is issuing pink slips to 350 former Horizon employees.The going has
also been tough for Bristol-Myers Squibb (BMS) — it reported low Q2 revenues and had to cut its full-year forecasts
as two of its top drugs, blood cancer treatment Revlimid and blood thinner Eliquis, saw a drop in sales due to competition
from generics. As a result, BMS laid off 48 employees in New Jersey in May, and plans to do away with another 100-odd employees soon.Nearly a year
after GSK spun off its consumer healthcare
business to create Haleon, there are reports that hundreds in the
United Kingdom and potentially thousands working globally for Haleon are at the risk of losing their jobs.
The restructuring is likely to save GBP 300 million (US$ 393 million) over the next three years.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Pfizer, Thermo Fisher, Novavax announce
layoffs due to declining Covid salesIn October, Pfizer had significantly lowered its full-year revenue forecast for 2023 due to reduced demand for its Covid products. The drug behemoth has also announced cost-cutting measures such as layoffs and expense cuts that will help save US$ 3.5 billion.During the same
month, Pfizer announced plans to retrench 791 employees at its Gladstone site in the US. A month
later, the company announced 800 job cuts, by reducing staff at its Kent (the UK), Michigan (the US), and Newbridge, Kildare (Ireland) sites.Thermo Fisher Scientific has been downsizing its workforce at
different locations since last year. The company manufactures Covid testing
kits. In our Q1 2023 update, we had mentioned that the company laid off around 500 employees across various locations in California between January 2022 and mid-April 2023.In a fresh wave
of job cuts announced in November, Thermo Fisher will layoff 97 employees in January 2024. The company plans to
close its facility in Alabama. Earlier in August, the company fired 205 staffers across two separate sites in Alachua, Florida, due to the relocation of development, manufacturing, and production activities from this site to their new Plainville, Massachusetts site.In November,
Covid vaccine maker Novavax announced second round of cost cuts for 2023 in order to reduce spending by US$ 300 million. In May, Novavax had reduced its
headcount by 25 percent to align its spending with the diminishing size of the Covid opportunity.Similarly, in
June, contract manufacturer Catalent said it plans to retrench 150 employees at its Bloomington, Indiana plant.
Catalent has been working closely with drugmakers to manufacture Covid vaccines
and therapies, and the job cuts are part of its post-pandemic restructuring
exercise.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Shifts in strategy, restructuring trigger layoffs at Takeda, PTC Therapeutics, ApellisIn May, Takeda had announced a shift in its R&D strategy, leading to 186 layoffs in Massachusetts and an additional 27 in San Diego. This announcement follows Takeda’s decision to discontinue discovery and pre-clinical efforts in AAV (adeno-associated virus) gene therapy and rare hematology.In a similar move
announced in September, rare disease drugmaker PTC Therapeutics announced retrenchment of 25 percent of its workforce as part of a broader restructuring initiated in May, involving discontinuation of several early-stage gene therapy development programs to focus on high-potential R&D programs. PTC plans to complete the process by January 15. In August, Apellis announced its plan to layoff
approximately 225 employees and divest two preclinical assets to
achieve US$ 300 million in savings as part of a significant corporate
restructuring program. This move aims to drive the growth of Syfovre, an
injectable form of pegcetacoplan that received FDA approval for geographic atrophy (GA) in February.During the same
month, Sage Therapeutics, a company working on novel therapies
for brain health disorders, announced 188 job cuts. These were part of a restructuring plan announced soon after FDA rejected its
drug Zurzuvae (developed along with Biogen) for major depressive disorder (MDD). The agency has approved the pill for postpartum depression, which is a much smaller market as compared to MDD.In September, 2seventy bio (a company spun out of bluebird bio) had announced plans to layoff about 40 percent of its workforce (i.e. axe 176 jobs) in order to lower costs and focus on the biotech firm’s cancer cell therapy — Abecma.In August, Emergent BioSolutions decided to cut 400 jobs and scale back operations at some of its
facilities, in an effort to move away from its CDMO business and pivot its focus on core products, such as
nasal spray Narcan and anthrax vaccines.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Our viewThe
biopharmaceutical industry is still grappling with the disruptions caused by
Covid. Some of the layoffs reflect a correction, as demand moves back to
pre-pandemic days. The others are either a fallout of disappointing quarterly
results, or the outcome of normal business restructuring and strategy shifts.While this
article was going to print, there was news that Kite Pharma, a subsidiary of Gilead focusing on cell therapies, is letting
go off 7 percent of its staff, or around 300 employees in order to improve
operational efficiency. Similarly, another genetic medicines company Generation Bio is firing 68 employees. Both these updates follow news that FDA is investigating the “serious risk” of cancer patients developing secondary blood cancer after undergoing chimeric antigen receptor T-cell (CAR-T) therapies. Incidentally, several startups focusing on cell and gene therapies (such as
Summation Bio, Oncurus Inc, Intergalactic Therapeutics), have had to shut operations due to lack of finance.As we bid
farewell to 2023, there are larger concerns of a slowdown in the global economy. For now, it seems
like 2024 may not be very different from 2023.
Impressions: 6651
After a year when
drug approvals by the US Food and Drug Administration
(FDA) slipped to the lowest since 2016, the first half of 2023 (H1 2023) saw a
62.5 percent growth in drug approvals by the Center for Drug Evaluation and Research (CDER) as compared with the same period last year. FDA’s CDER approved 26 new drugs
in 2023, up from 16 in H1 2022. Approvals of biologics by the FDA surpassed full-year totals for many of the past 20 years. Nine new biologics were approved by FDA’s Center for Biologics Evaluation and Research (CBER) in H1 2023, as against just eight
for 2022.While FDA approved more drugs, authorizations by the European Medicines Agency (EMA) also increased but drug approvals by Health Canada dipped by approximately 50 percent. In the first half of 2023, EMA authorized 14 drugs, as opposed to 10 in H1 2022. Similarly, Health Canada approved 13 drugs against 25 in H1 2022.Among the
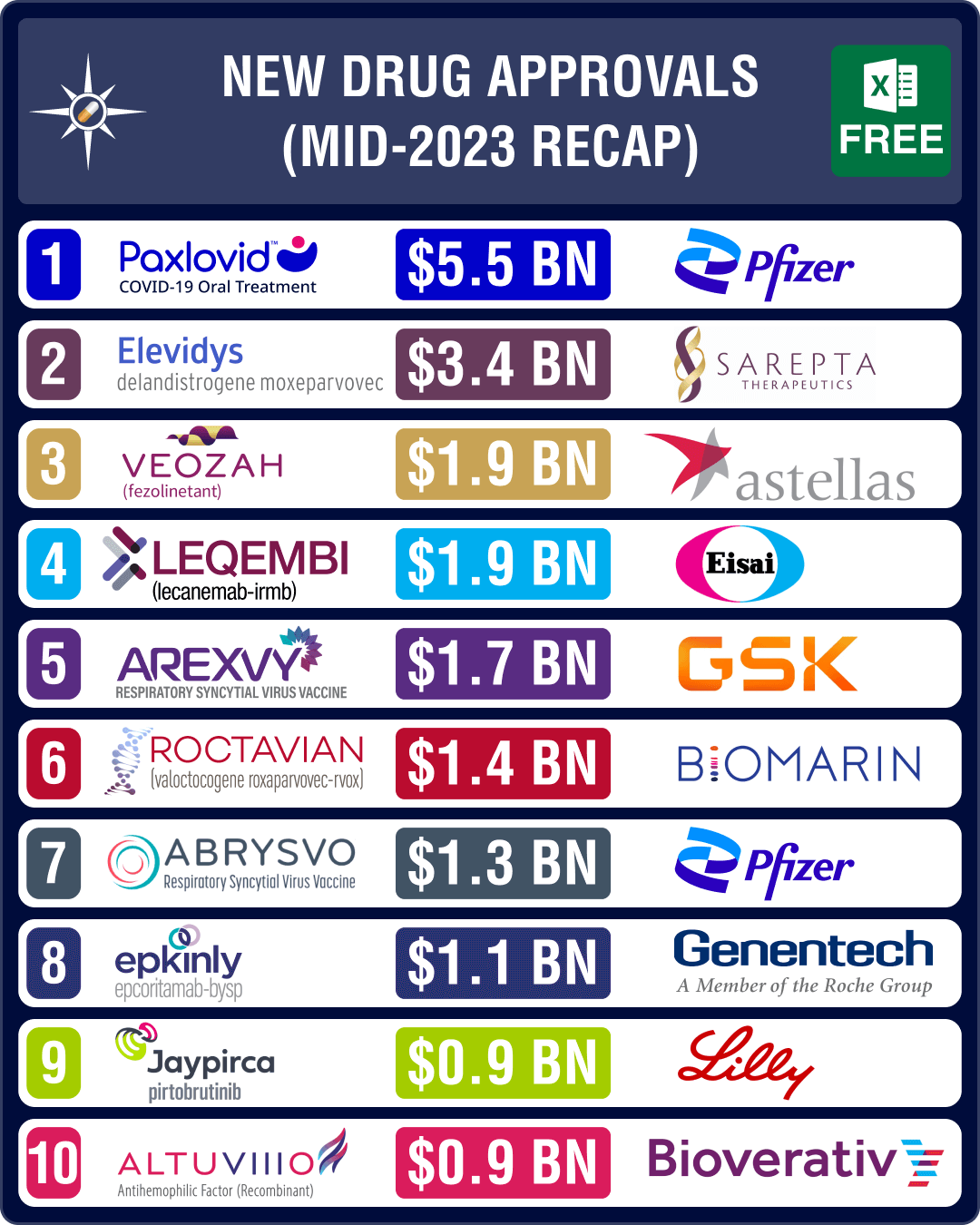
promising drugs approved in H1 2023 are Biogen and Eisai’s Alzheimer’s drug Leqembi (lecanemab), Astellas Pharma’s drug for hot flashes (associated with
menopause) Veozah, AbbVie and Genmab’s blood cancer drug Epkinly and, GSK and Pfizer’s RSV vaccines Arexvy and Abrysvo, respectively.View New Drug Approvals in first half of 2023 with Estimated Sales (Free Excel Available)GSK, Pfizer’s RSV vaccines to generate over US$ 1 billion by 2028The highlight of H1 2023 has been the approval of vaccines for respiratory syncytial virus (RSV), a contagious virus that causes infections of the respiratory tract, and can cause severe illness in older adults and infants. In May 2023, FDA approved GSK’s Arexvy, respiratory syncytial virus (RSV) vaccine for people aged 60 and older, making it the first vaccine approved in the US that protects against the disease. The drug is expected to generate US$ 1.73 billion in peak sales by 2028.Weeks later, the agency approved Pfizer’s Abrysvo, another RSV vaccine for older adults. The vaccine is expected to be available
in the third quarter of 2023. In late August, Pfizer also bagged an FDA nod for
use of Abrysvo to prevent RSV in infants by vaccinating pregnant women. The
drug is expected to generate US$ 1.31 billion in peak sales by 2028.View New Drug Approvals in first half of 2023 with Estimated Sales (Free Excel Available) Leqembi, Veozah, Epkinly likely to join ‘over US$ 1 billion club’ by 2028In March, the US health regulator suggested stricter trials for accelerated approval of cancer drugs. The proposed recommendation followed criticism over the approval of Biogen and Eisai’s Alzheimer’s treatment Aduhelm through this pathway in June 2021.Contrary to this
stand though, FDA had approved another Alzheimer’s drug from Biogen and Eisai — Leqembi (lecanemab) — through the same pathway in January 2023. Leqembi is an amyloid beta-directed antibody indicated for the treatment of Alzheimer’s disease. The drug is expected to generate US$ 1.9 billion in peak sales by 2028. Later in July, FDA expanded the accelerated approval of lecanemab to a traditional approval for early-stage Alzheimer’s disease.The US regulator
approved several potential blockbuster drugs during the month of May. It
granted full approval to Pfizer’s Covid-19 oral antiviral pill Paxlovid (nirmatrelvir and ritonavir) during the month, which had been
granted an emergency use authorization during the heydays of the pandemic. Paxlovid is the first oral antiviral pill for Covid-19. With the pandemic behind us, Paxlovid’s sales have been waning, though it is still projected to yield approximately US$ 5.5 billion in sales by 2028.The other
important drug approved in May is Japanese drugmaker Astellas Pharma’s oral drug Veozah (fezolinetant) for the treatment of moderate to severe vasomotor symptoms (VMS), or hot flashes associated with menopause. The drug will cost US$ 550 for a month’s supply. Veozah is expected to generate US$ 1.9 billion in sales by 2028.FDA also granted
accelerated approval to AbbVie and Genmab’s blood cancer therapy Epkinly (Epcoritamab-Bysp) in May. It
has bagged approval to treat patients with diffuse large B-cell lymphoma
(DLBCL), and is likely to emerge as a US$ 1.1 billion product.View New Drug Approvals in first half of 2023 with Estimated Sales (Free Excel Available) Sarepta, Biomarin’s gene therapies bag FDA nod; Lilly’s Jaypirca okayed for lymphomaIn June, FDA
approved Sarepta’s Elevidys and Biomarin’s Roctavian. Elevidys bagged accelerated approval in Duchenne muscular dystrophy (DMD)
patients aged four to five years. This is the first gene therapy approved by
the FDA for the treatment of certain patients with DMD. Elevidys is expected to
generate US$ 3.36 billion in peak sales by 2028.BioMarin’s one-time gene therapy – Roctavian – has been approved by the FDA to treat severe hemophilia A, a
rare bleeding disorder. Priced at US$ 2.9 million, Roctavian is the first gene
replacement therapy to be approved for the condition. Roctavian is expected to
generate US$ 1.43 billion in peak sales by 2028. This approval follows another
approval granted in November 2022 for the first gene therapy to treat adults with hemophilia B — CSL Behring and uniQure’s Hemgenix.In January, Lilly’s Jaypirca (pirtobrutinib) bagged FDA nod, thereby becoming the first and only non-covalent, BTK Inhibitor for the
treatment of adult patients with relapsed mantle cell lymphoma which did not
respond to other BTK Inhibitors. Jaypirca is expected to generate US$ 0.91
billion in peak sales by 2028.In March, FDA approved Pfizer’s Zavzpret (zavegepant) as the first and only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray for the acute treatment of migraine. Zavzpret is expected to generate US$ 0.649 billion in peak sales by 2028.Another important FDA approval was granted to GSK’s drug Jesduvroq — it is now the first oral treatment for anemia caused by chronic kidney disease in adults who have been on dialysis for at least four months.View New Drug Approvals in first half of 2023 with Estimated Sales (Free Excel Available) FDA rejects Lilly’s donanemab, Alvotech/Teva’s Humira biosimilarIn January, when
the FDA had approved Leqembi, the agency had rejected Eli Lilly’s bid for an accelerated approval pathway for its experimental Alzheimer’s disease drug donanemab. The agency had cited a lack of participants who received continuous treatment with donanemab for at least 12 months as the reason for its decision. However, Lilly
announced positive late-stage trial
results on donanemab in July — it slowed cognitive decline by 35 percent compared to a placebo in a late-stage trial. An FDA decision on the drug is likely around 2023-end or in early 2024.Similarly, FDA turned down Lilly’s mirikizumab for the treatment of ulcerative colitis. The agency also rejected a BLA for Eli Lilly’s ulcerative colitis drug mirikizumab over manufacturing concerns. Besides these, FDA also rejected Alvotech/Teva’s Humira biosimilar — AVT02 — for a second time this year.View New Drug Approvals in first half of 2023 with Estimated Sales (Free Excel Available) Our viewAfter the
pandemic, business is back to normal at the FDA. The number of CDER approvals
in the first half of this year are approximately 70 percent of the approvals in
2022. We anticipate more approvals in the upcoming months. If the FDA sustains
this pace, approvals this year could potentially double those of the previous
years.
Impressions: 3537