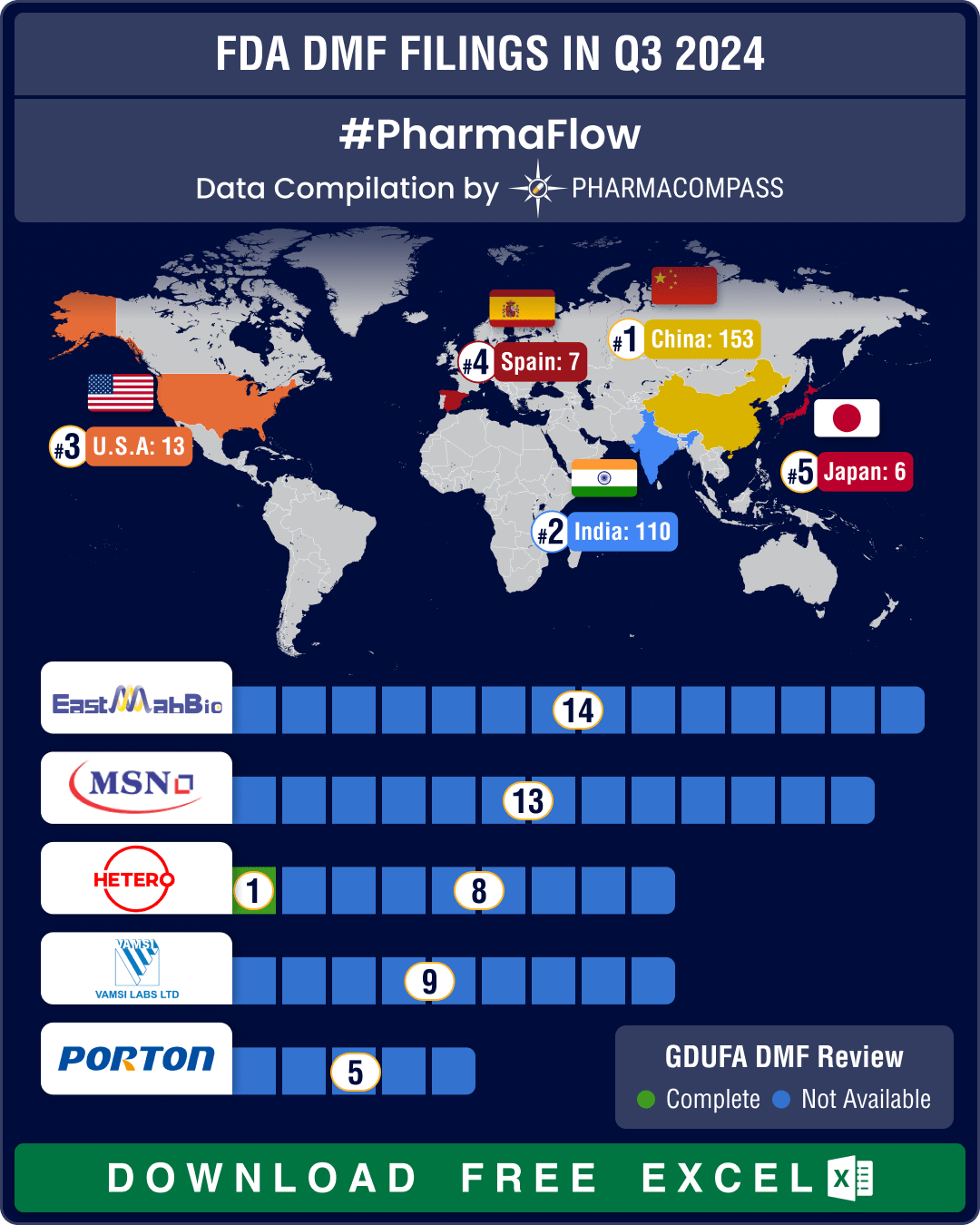
DMF filings hit all-time high in Q3 2024; China tops list with 58% increase in Type II submissions
Drug Master Files, or DMFs, are confidential documents that play a crucial role in the pharmaceutica
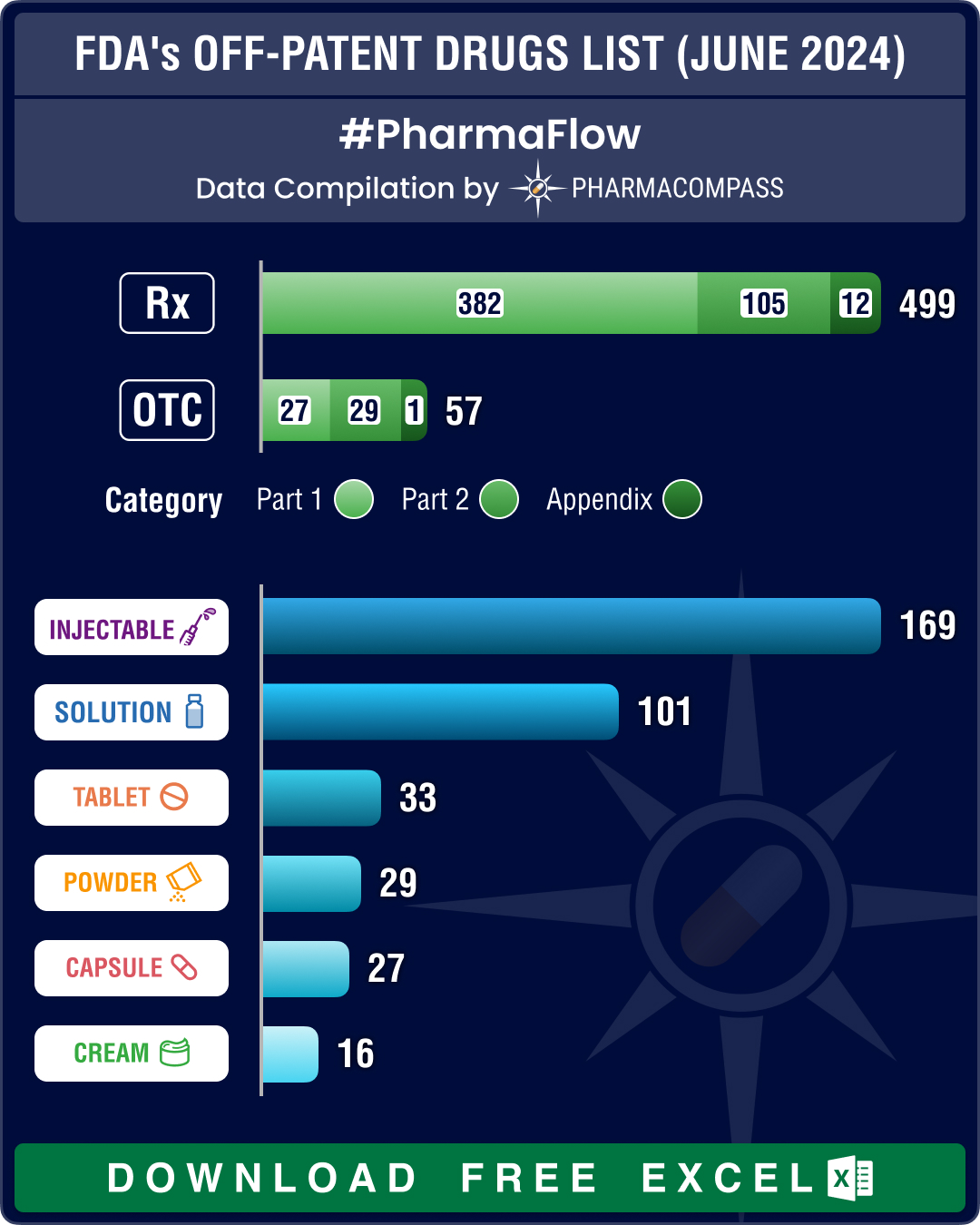
FDA’s June 2024 list of off-patent, off-exclusivity drugs sees rise in cancer, HIV treatments
This week PharmaCompass brings to you key highlights of the US Food and Drug Administration’s


 Market Place
Market Place Sourcing Support
Sourcing Support