By PharmaCompass
2022-02-17
Impressions: 6121
The year 2021 was eclipsed by the Covid-19 pandemic. In our update for the first half of 2020, we had mentioned that Covid-19 has not slowed down the speed at which generic active pharmaceutical ingredient (API) manufacturers were submitting Drug Master Files (DMFs) to the US Food and Drug Administration (FDA). That trend continued in 2021, when the speed of DMF submissions to the agency remained similar to that witnessed in the previous years.
In fact, Type II DMFs, or DMFs for active pharmaceutical ingredients (APIs), were higher in 2021 as compared to previous years. In the first quarter, FDA received 164 Type II DMF submissions, which rose to 165, 166 and 172 submissions over the next three quarters. In all, 667 Type II DMFs were filed in 2021, as opposed to 662 in 2020, 633 in 2019 and 644 in 2018.
DMFs are submissions made to the FDA by manufacturers who provide the agency with confidential, detailed information about facilities, processes or articles used in manufacturing, processing, packaging and storing of human drug products.
Overall, 2021 saw a total of 913 DMFs (Type II, III, IV and V) being submitted. In comparison, FDA had received 931 DMF submissions in 2020, 894 in 2019 and 979 in 2018.
View FDA DMF Filings in 2021 (Power BI Dashboard, Free Excel Available)
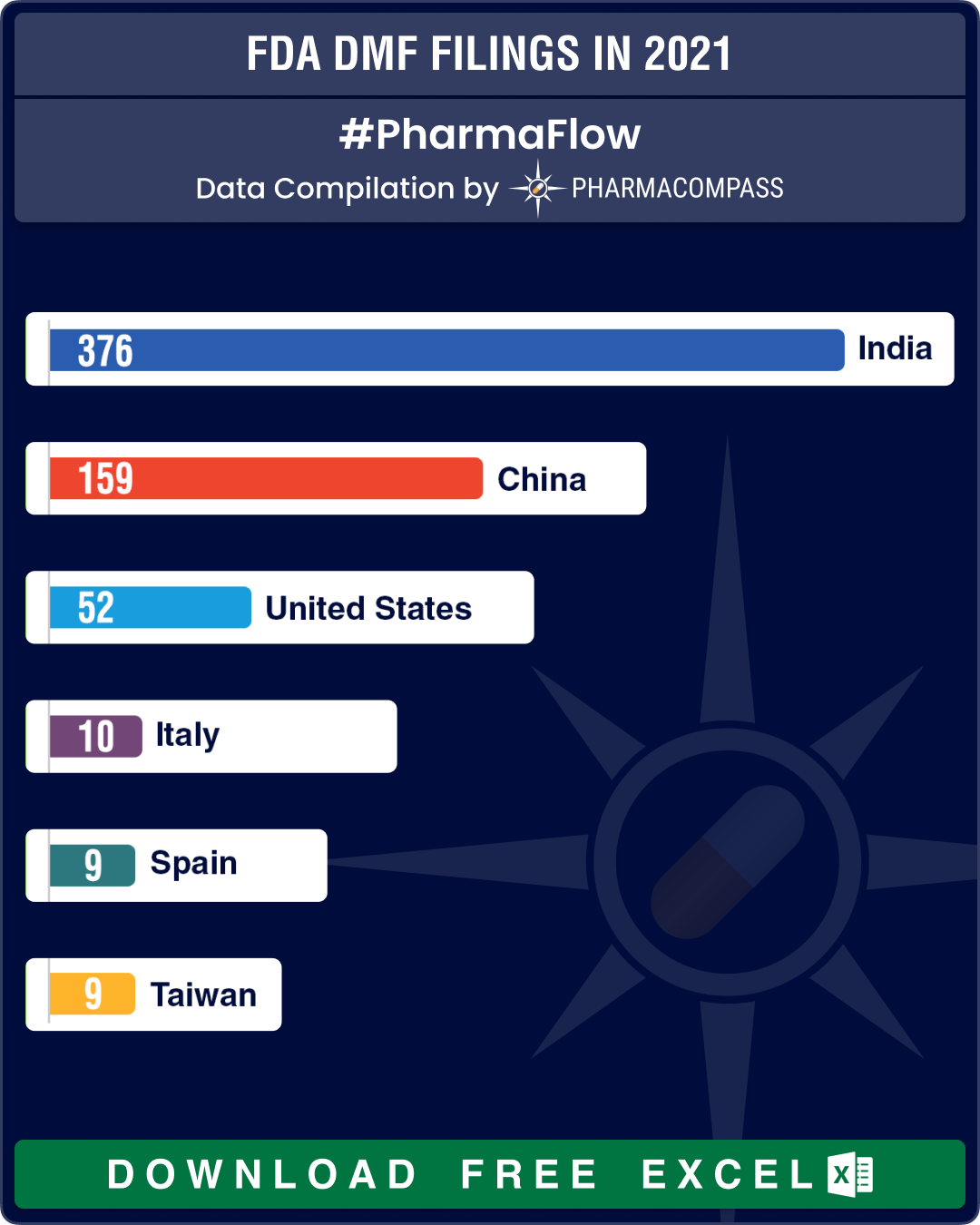
India continues to lead DMF filings, followed by China
Country-wise data on DMF filings at the FDA tells us the potential of a country in the field of pharmaceuticals. At the company-level, with each DMF filing, a firm commits itself to manufacturing drugs in a facility that is aligned to the FDA’s rules and regulations.
This year too, DMFs filed from India and China were significantly higher than those from other countries. Expectedly, India continued to lead with 376 DMF filings. Submissions from India were over twice that of DMF filings from China (at 159). This is not surprising since the two countries have the maximum number of API manufacturing facilities registered with the FDA.
As compared to this, the United States had 52 DMF filings, Italy had 10, Spain and Taiwan had 9 each, and countries like Canada, Israel, Japan and UK had five DMF filings each.
View FDA DMF Filings in 2021 (Power BI Dashboard, Free Excel Available)
India’s MSN Labs leads DMF count
As in the past, India's MSN Laboratories continued to lead the DMF filings by a single company with 43 submissions. MSN was followed by five other Indian companies — Dr. Reddy’s Laboratories filed 15 submissions, Hetero Group and Aurobindo Pharma 14, Metrochem API 13 and Aurore Life Sciences filed 12 DMF submissions.
The only Chinese company in the top 10 by DMF count was Brightgene Bio-Medical Technology Limited with nine DMF submissions.
The maximum number of DMF filings were for semaglutide (eight), followed by favipiravir (seven), apalutamide (six), sitagliptin phosphate (six) and tofacitinib citrate (six). Others like acalabrutinib, elagolix sodium, lenalidomide, liraglutide and pantoprazole sodium had five DMF filings each.
View FDA DMF Filings in 2021 (Power BI Dashboard, Free Excel Available)
Slow assessment review, higher GDUFA fee
Although there were 667 Type II DMFs filed with the FDA, only 194 (or 29 percent) had their review completed. The GDUFA (short for Generic Drug User Fee Amendments) fee associated with a DMF assessment review for 2021 was considerably higher — at US$ 69,921 — as opposed to US$ 57,795 for 2020. For FY 2022, the GDUFA fee has been revised upward to US$ 74,952 (an increase of US$ 5,031).
There are 42 products for which a DMF was filed for the first time. Among the patented products which should expect generic competition are avatrombopag, encorafenib, esketamine hydrochloride, siponimod fumaric acid, tedizolid phosphate and vorapaxar sulfate.
In fact, DMFs were also filed for products that are yet to receive an FDA approval. Some of these products are imeglimin, aviptadil, gimeracil, linzagolix choline, meglumine antimoniate, roluperidone hydrochloride and teneligliptin.
View FDA DMF Filings in 2021 (Power BI Dashboard, Free Excel Available)
Our view
The Covid-19 pandemic revealed how the global supply chain for pharmaceuticals is excessively dependent on India and China. As a result, many countries across the world are making investments into expanding their API production capacities. This should translate into more Type II DMF filings from countries other than India and China.
Moreover, as the pandemic begins to wane and the FDA increases its inspections — both domestic and international — compliance issues are bound to increase. The US is planning to run a pilot program soon that will test a system of unannounced inspections in India and China. Companies in both India and China will need to increase their focus on compliance if they wish to continue to be major contributors to the global supply chain for pharmaceuticals. We can certainly expect more regulatory news in 2022.
View FDA DMF Filings in 2021 (Power BI Dashboard, Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA DMF FILINGS IN 2021 by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








